Humanised antibodies to epidermal growth factor receptor
A humanized and human antibody technology, applied in the direction of antibodies, anti-receptors/cell surface antigens/cell surface determinant immunoglobulins, antibody mimics/scaffolds, etc., can solve the problem of enhancing the ability of cell growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1 - Construction of chimeric antibodies from α340
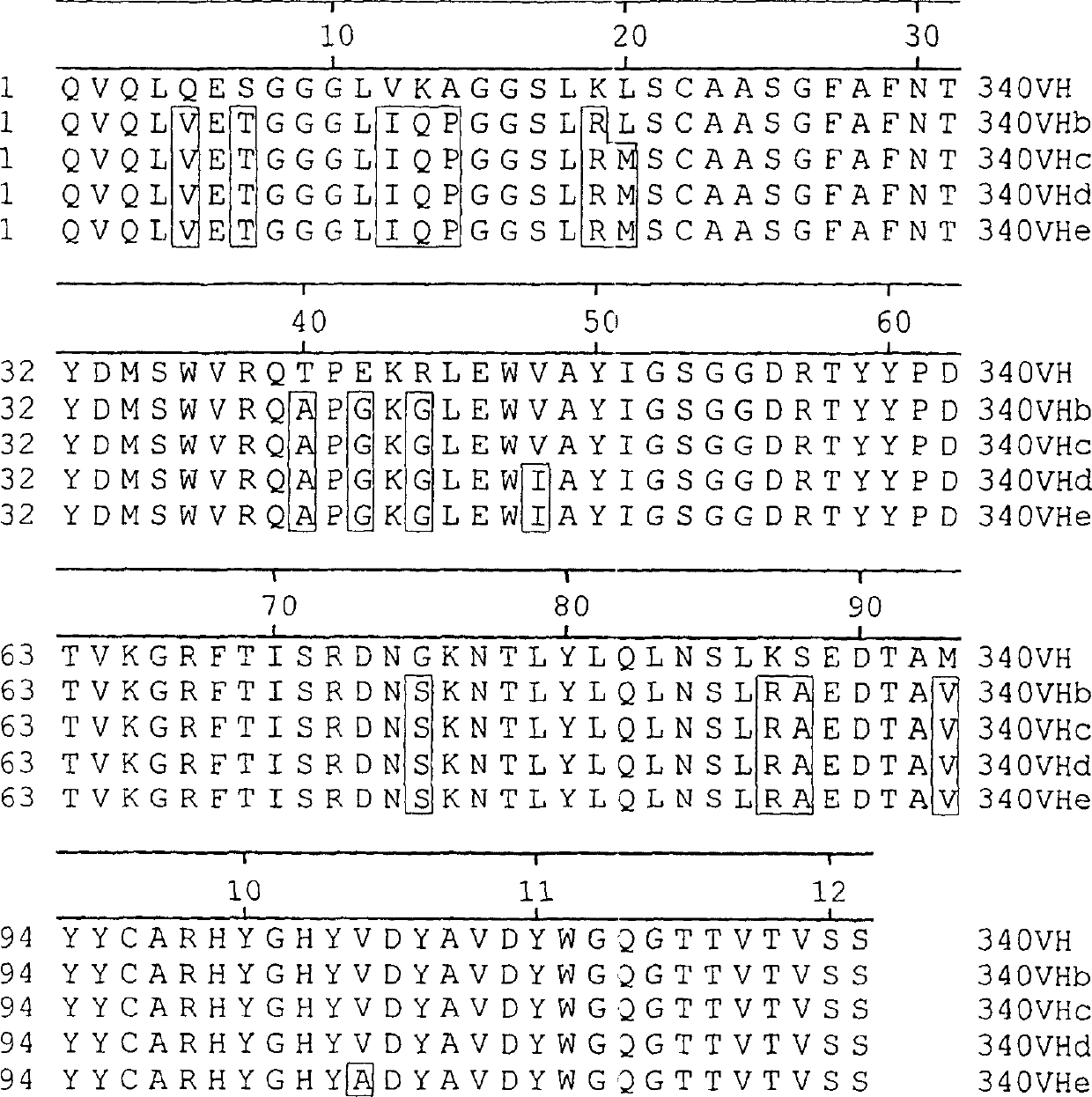
[0078] Use the Qiagen RNeasy kit according to the instruction manual from 5 × 10 6 Total RNA was isolated from hybridoma α340 cells (Durrant et al., Prenatal Diagnostics, 14, 131, 1994). RNA was converted to cDNA using Promega (Southampton, UK) reverse transcriptase, buffer and dNTPs. Variable heavy chain (V H ) and light chain (V L ) cDNAs were amplified with the primer set of the method of Jones and Bendig (Bio / Technology, 9, 188, 1991). The amplified DNA was gel purified and cloned into vector pGem T Easy (Promega). PCR products were bidirectionally sequenced using an Applied Biosystems Automated Sequencer Model 373A (Applied Biosystems, Warrington, UK). get V H and V L The DNA sequence is shown in Figure 1 and the protein sequence is shown in Figure 2 (when used here V L with V K same).
[0079] The positions of the complementarity determining regions (CDRs) were determined with reference to o...
Embodiment 3
[0090] Example 3 - Construction of deimmunized antibody sequences
[0091] Deimmunized variable regions were constructed by overlapping PCR recombination. cloned murine V H and V K The gene serves as a template for mutagenizing the framework regions to the desired deimmunized sequences. Several sets of mutagenic primer pairs containing the region to be altered are synthesized.
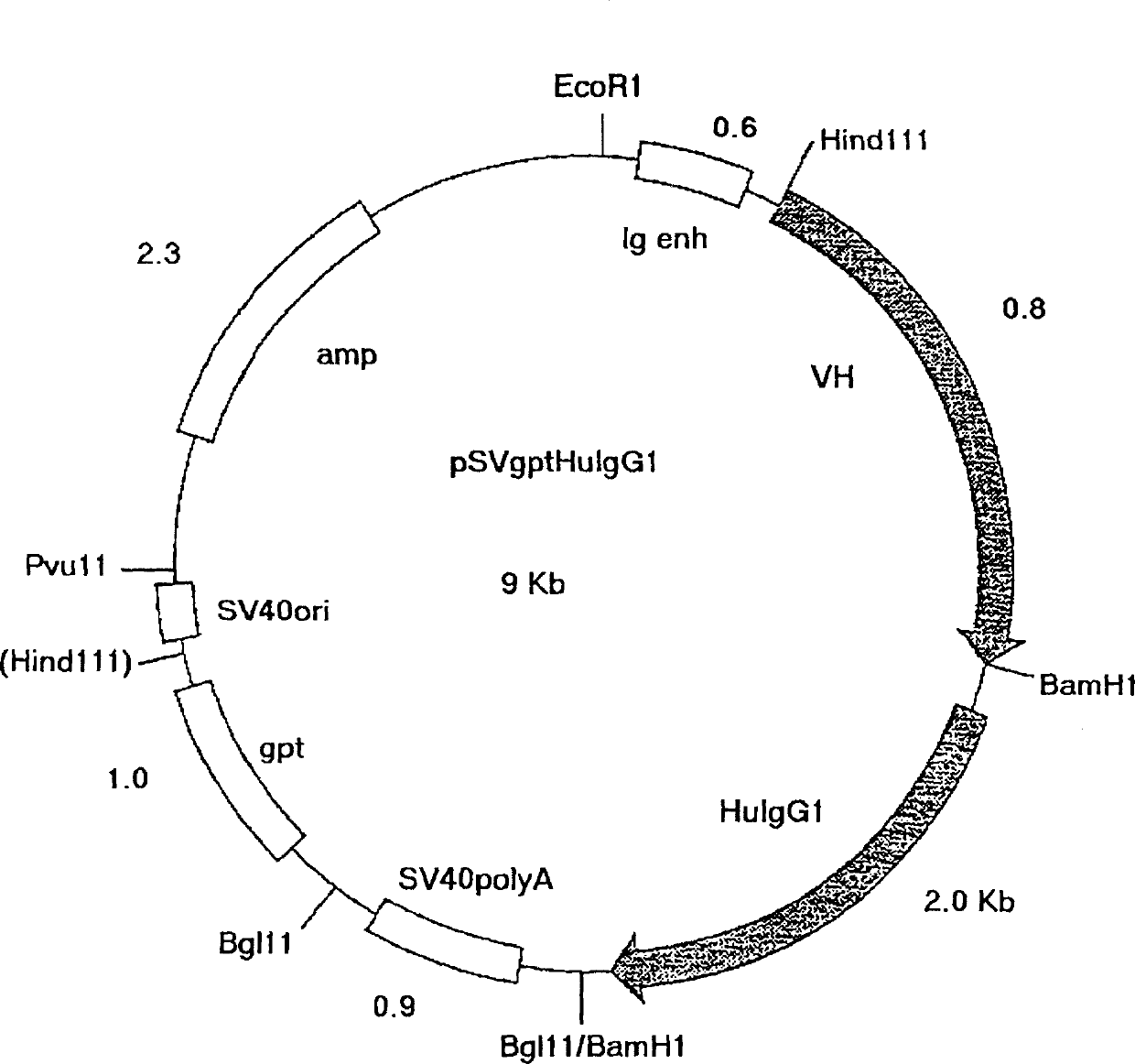
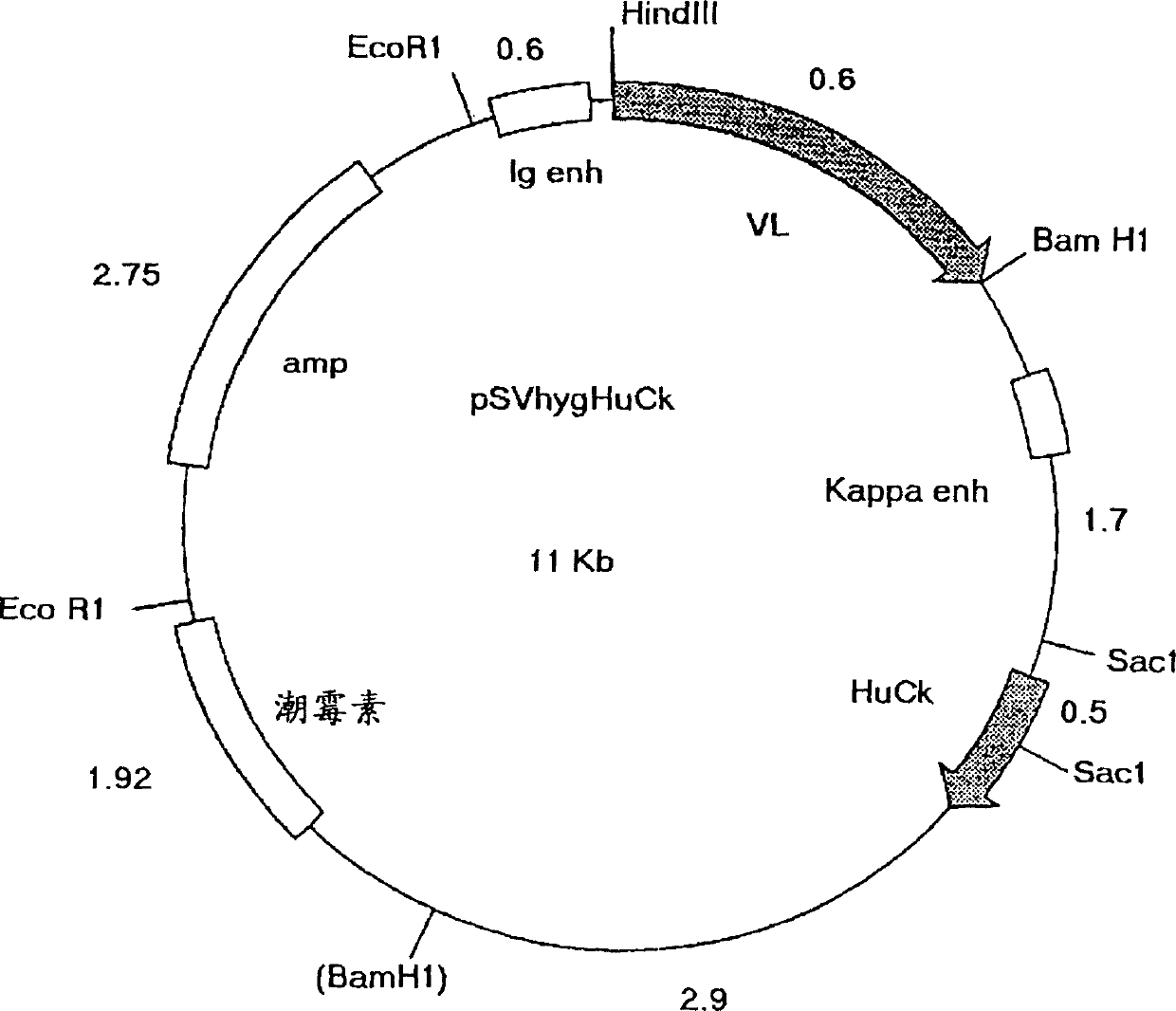
[0092] The application of mutagenic primer pairs requires an annealing temperature of 48°C-50°C. All amplifications were performed using pfu turbo proofreading polymerase (Stratagene, La Jolla, California). #340 Chimeric V H and V K The construct (respectively corresponding to the vector VH / VK-PCR1 with the insert of the variable region shown in Figure 6 and Figure 7) was used as a template to introduce the 5' flanking sequence, including the leading signal peptide, leading intron and Mouse heavy chain immunoglobulin promoter, and 3' flanking sequences, including splice sites and intronic sequen...
Embodiment 5
[0099] Example 5 - Production and detection of deimmunized α340 antibody
[0100] Antibody was purified from 500ml-11 standing culture by stirring overnight with 0.5ml ProSepA (Bioproeessing Ltd). Prosep A was then isolated by affinity chromatography. Antibodies were eluted with 0.1 M glycine (pH 3.0), neutralized, and dialyzed overnight against PBS. Purified antibody preparations were filter sterilized and stored at 4°C. For human IgG, the concentration of purified antibody was determined by ELISA.
[0101] Detection of α340DI antibody variants (different forms of deimmunized antibodies prepared with variable regions as shown in Figure 6 and Figure 7 and constant regions on vectors pSVgpt and pSVhyg, respectively) and A431 cells (ECCAC No. 85090402) by ELISA combination. These epithelial monolayers overexpress epidermal growth factor receptor (EGFR) and are therefore suitable for assaying α340 binding to EGFR antigen. A431 cells were grown to confluency in 96-well plates...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com