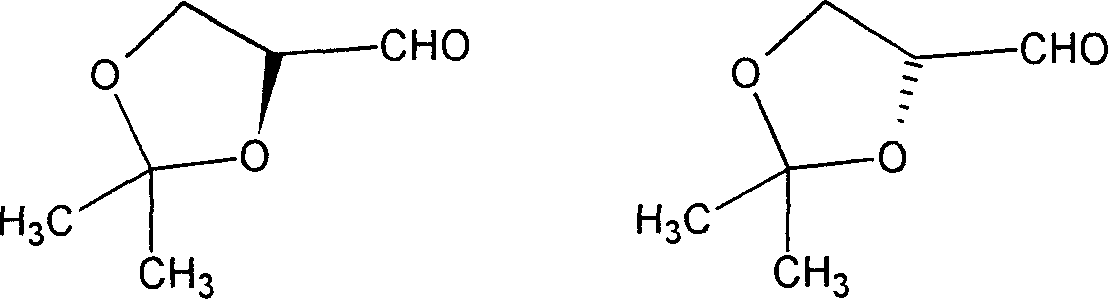Process for producing L-(S) propylidene glycerin aldehyde solution
A technology of propylidene glycerol and its production method, which is applied in the field of production of L-(S)-propylidene glyceraldehyde solution, and can solve the problems of instability, high production cost and poor purity of L-(S)-propylidene glyceraldehyde. Problems, to achieve the effect of improving equipment efficiency and labor efficiency, low cost, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of L-gulonic acid-1,4-lactone
[0033] Dissolve 23.1 grams of vitamin C in 170 milliliters of water, add 2.2 grams of 5% palladium / carbon catalyst, at 50 ° C and 5.05 × 10 5 Catalyzed hydrogenation under Pa for 24 hours, filtered to remove the catalyst, and evaporated the water under reduced pressure to obtain L-gulonic acid-1,4-lactone, which was recrystallized with a mixed solvent of methanol and ethanol to obtain 22 grams of the product with a yield of 95%. , melting point 182-183.5°C; [α]- is +55.2° (c=1, H 2 O);
[0034] IR(KBr)1770cm -1 ;
[0035] 1 H-NMR (Me 2 SO-d 6 ): δ=
[0036] 5.80 (d, 1, OH), 5.30 (d, 1, OH), 4.95 (d, 1, OH) 4.45-4.07 (m, 3), 4.00-3.35 (m, 3), 4.00-3.35 (m, 3).
[0037] Synthesis of 5,6-O-isopropylidene-L-gulonic acid-1,4-lactone
[0038]Dissolve 221.6 grams of L-gulonic acid-1,4-lactone in 2000 milliliters of N,N-dimethylformamide, cool to 10°C, add 1.8 grams of p-toluenesulfonic acid while stirring, and keep the temper...
Embodiment 2
[0045] Production of L-gulonic acid-1,4-lactone
[0046] Dissolve 200 kg of vitamin C in 1000 liters of water, add 20 kg of 10% palladium / carbon catalyst, at 50 ° C and 5.05 × 10 5 Catalytic hydrogenation under Pa for 24 hours, remove the catalyst by filtration, distill off the water under reduced pressure to obtain a white solid which is the crude product of L-gulonic acid-1,4-lactone, which is recrystallized with a mixed solvent of methanol and ethanol to obtain a white cube Crystalline L-gulonic acid-1,4-lactone 196 kg, yield 98%, melting point 183-183.5°C, [a] 25 is +55.5° (c=1, H 2 O).
[0047] Synthesis of 5,6-O-isopropylidene-L-gulonic acid-1,4-lactone
[0048] Dissolve 190 kg of L-gulonic acid-1,4-lactone in 1500 liters of DMF, cool to 10°C, add 1.8 kg of p-toluenesulfonic acid while stirring, and add 2,2-dimethyl dropwise while keeping the temperature below 10°C 150 kg of oxypropane, continue to stir at room temperature for 24 hours, then add 220 kg of sodium carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com