Medicinal preparation containing erythromycine ethylsuccinate
A technology of erythromycin ethylsuccinate and pharmaceutical preparations, applied in the directions of medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., can solve the problem of successful development of parenteral administration formulations of erythromycin ethylsuccinate. and other problems, to achieve the effect of rapid curative effect, high safety and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
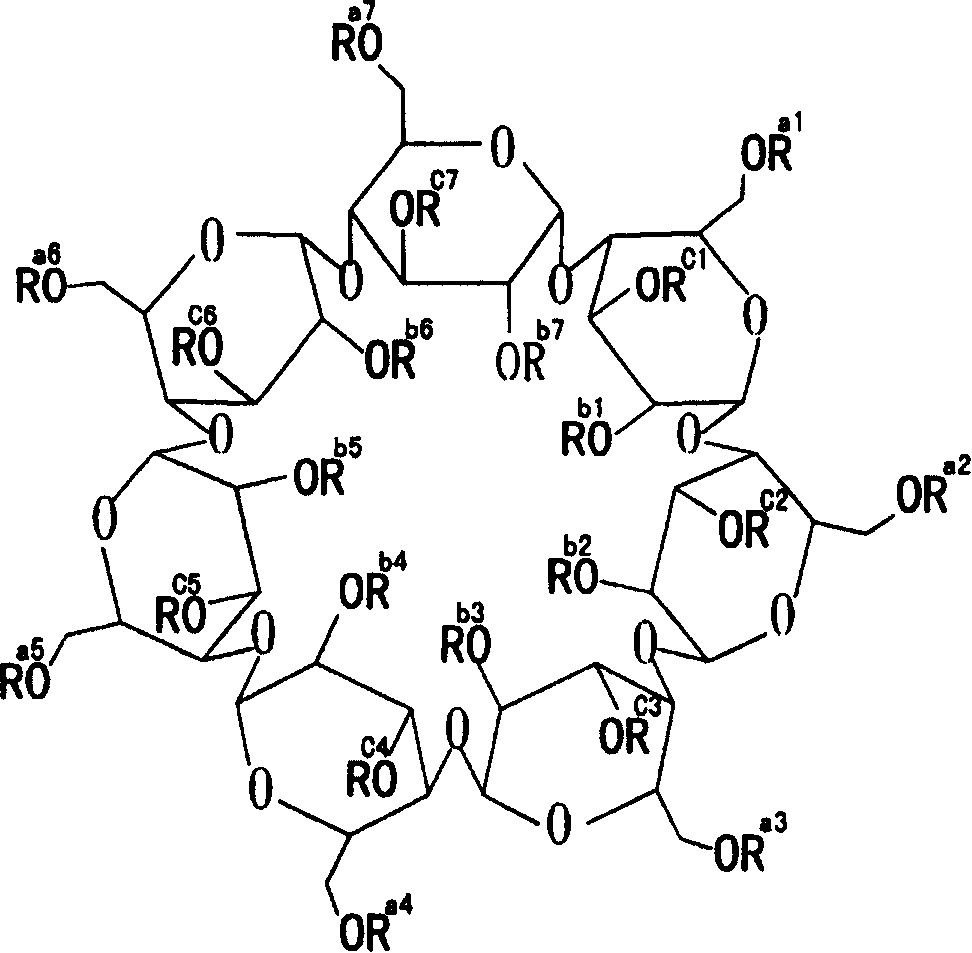
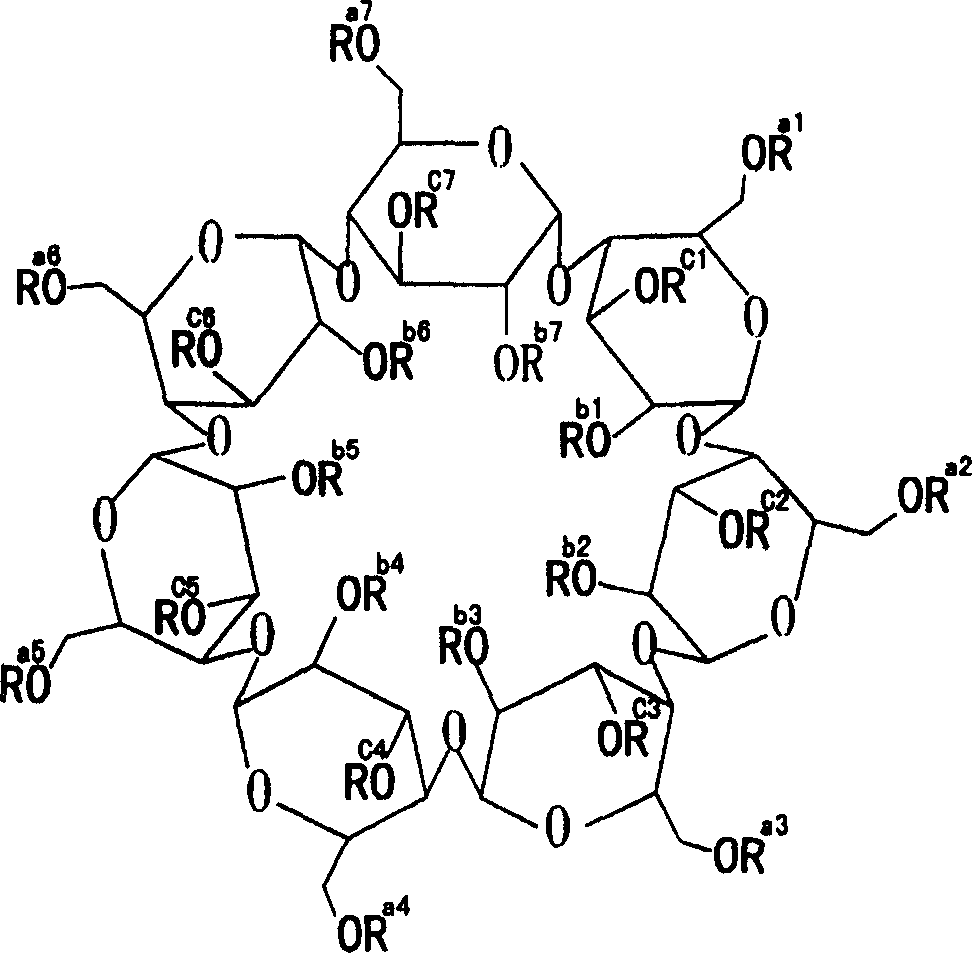
[0017] In the following examples, hydroxypropyl beta cyclodextrin has an average degree of substitution of hydroxypropyl groups per cyclodextrin molecule of 5.5. The prepared medicaments are all water-soluble powders for intravenous administration or intramuscular administration of erythromycin ethylsuccinate.
[0018] component
[0019] Add hydroxypropyl beta-cyclodextrin to 5ml of water under stirring conditions, and continue to stir until it is completely dissolved; add ethylsuccinate and stir until it is completely dissolved; microwave treatment in an ice bath for 2 minutes; the resulting solution is sterilized with 0.2 mm nylon membrane filter; add medicinal glucose powder, stir until dissolved; after freeze-drying, it can be packaged and used.
[0020] component
[0021] Add hydroxypropyl beta-cyclodextrin to 5ml of medicinal absolute ethanol under stirring conditions, and continue to stir until completely dissolved; add ethylsuccinate and stir until ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com