High steric-hindered amino-methano-phenol organic zinc compounds and process for preparing same
A technology of hindered aminomethylene phenol and a synthesis method, applied in the direction of zinc organic compounds and the like, can solve the problems of difficult preparation, price, poor selectivity, environmental problems and the like, and achieves the effects of mild reaction conditions, simple steps and good catalytic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
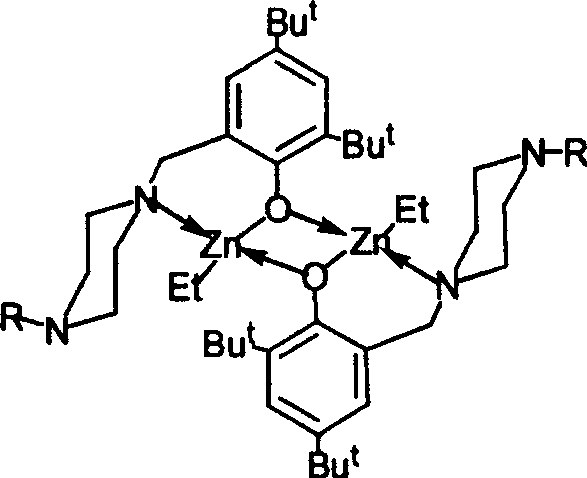
[0016] 实施例1:高位阻氨亚甲基酚有机锌化合物(I)的合成、表征及结构
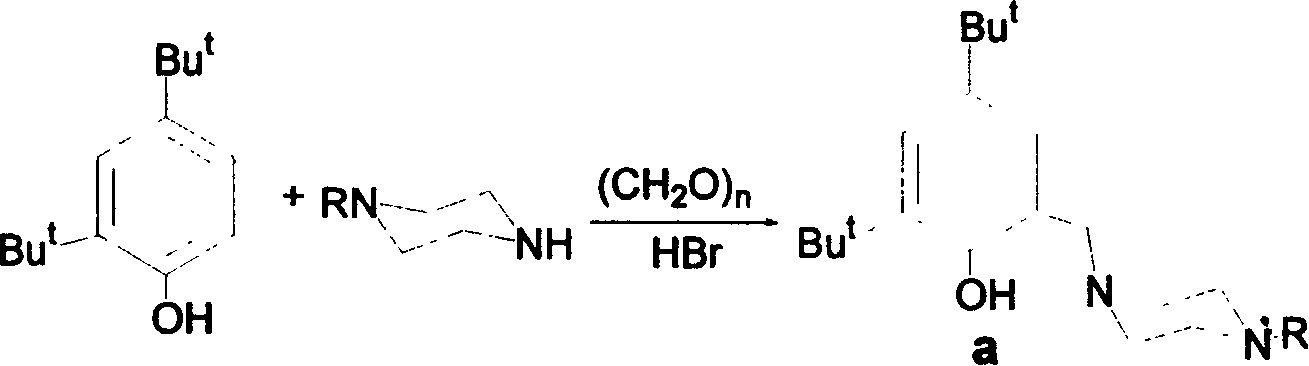
[0017] 将1-甲基哌嗪(4.96g,50mmol),多聚甲醛(1.80g,60mmol)和2,4-叔丁基苯酚(10.32g,50mmol)溶于50mL乙醇中。在氮气保护下加热回流20小时,冷却至室温。加入氢溴酸(8mL,48%水溶液)得胶状物,将该胶状物真空干燥,乙醇洗涤得白色固体。将上述所得白色固体溶于水中,用碳酸氢钠中和后用氯仿(30mL×3)萃取,合并有机并有机相,真空除去溶剂得无色晶体状配体13.69克,产率86%。
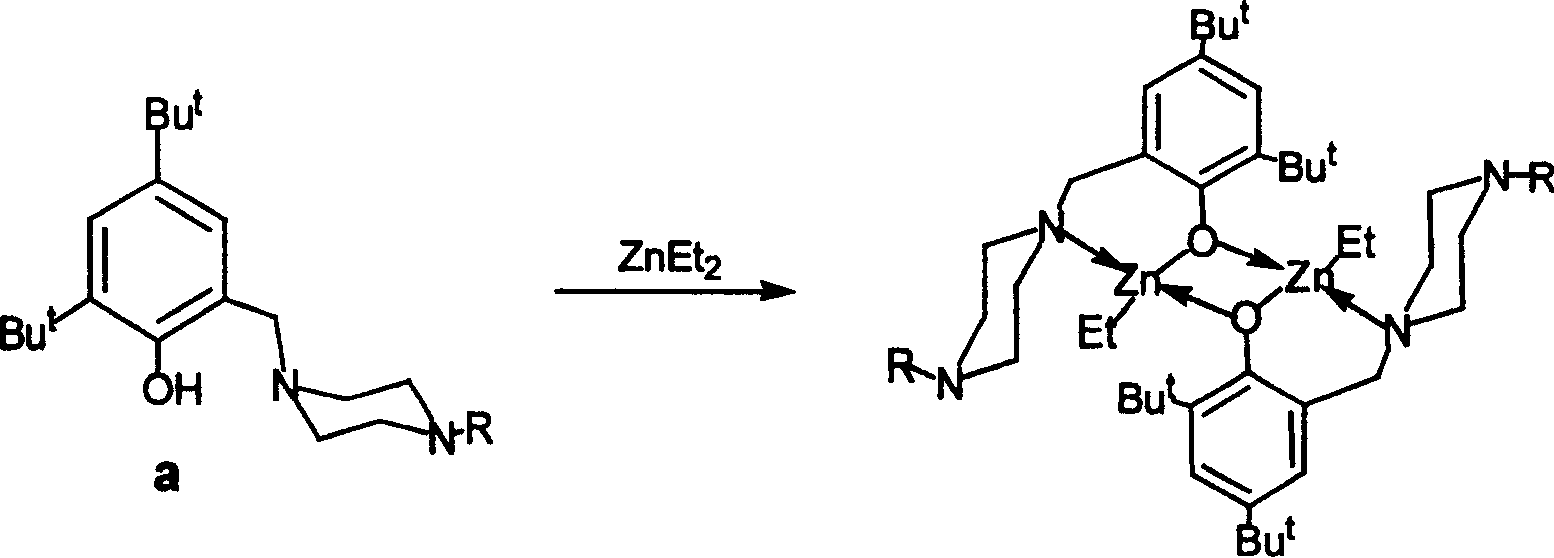
[0018] 将二乙基锌(3.26mL,1M的Et 2 O溶液,3.26mmol)在-35℃搅拌下用注射器慢慢滴加到盛有上述配体(1.04g,3.26mmol)的乙醚(40mL)溶液中,反应液缓慢升至室温,再继续搅拌16 Hour. 过滤得到白色沉淀并用正己烷洗涤后溶入四氢呋喃和正己烷的混合溶剂中,真空浓缩,保存在-25℃12小时得无色晶体状目标化合物(I)1.55克,产率87%。
[0019] NMR data 1 H NMR(C 6 D. 6 ):δ(ppm)0.51(q,2H,CH 3 CH 2 ),1.30(t,3H,CH 3 CH 2 ),1.39(s,9H,CMe 3 ),1.45(s,9H,CMe 3 ), 1.97 (s, 3H, CH 3 ),2.08,2.16(br,4H,4H,N(CH 2 CH 2 ) 2 N),3.56(s,2H,Ar-CH 2 ),6.99(s,1H,Ar-H),7.54(s,1H,Ar-H); 13 C NMR:δ(ppm)3.43(CH 3 CH 2 ),13.02(CH 3 CH 2 ),29.78(CMe3),32.02(CMe 3 ),34.05(CMe 3 ),35.60(CMe 3 ),46.11(Me),53.88,55.20(N(CH 2 CH 2 ) 2 N),65.34,(Ar-CH 2 ),119.36,124.72,126.16,135.74,137.69,162.06(Ar)。
[0020] 元素分析:理论徝C,64.14;H,9.30;N,6.80;实测徝C,74.06;H,9.23;N,6.98。 ...
Embodiment 2
[0025] 实施例2:高位阻氨亚甲基酚有机锌化合物(II)的合成、表征及结构
[0026] 1-苯基哌嗪(8.11g,50mmol),多聚甲醛(1.80g,60mmol)和2,4-叔丁基苯酚(10.32g,50mmol)溶于50mL乙醇中。在氩气保护下加热回流20小时,冷却至室温。加入氢溴酸(8mL,48%水溶液)得胶状物,将该胶状物真空干燥,乙醇洗涤得白色固体。将上述所得白色固体溶于水中,用碳酸氢钠中和后用氯仿(30mL×3)萃取,合并有机相,真空除去溶剂得无色针状晶体状配体15.98克,产率84%。
[0027] 将二乙基锌(3.26mL,1M的Et 2 O溶液,3.26mmol)在-35℃搅拌下用注射器慢慢滴加到盛有上述配体(1.08g,3.26mmol)的乙醚(40mL)溶液中,反应液缓慢升至室温,再继续搅拌16 Hour. 过滤得到白色沉淀并用正己烷洗涤后溶入四氢呋喃和正己烷的混合溶剂中,真空浓缩,保存在-25℃12小时得无色晶体状目标化合物(II)1.30克,产率84%。
[0028] 核磁数据: 1 H NMR(C 6 D. 6 ):δ(ppm)0.47(q,2H,CH 3 CH 2 ),1.30(t,3H,CH 3 CH 2 ),1.40(s,9H,CMe 3 ),1.71(s,9H,CMe3 ), 2.58-3.00 (br, 8H, N (CH 2 CH 2 ) 2 N), 3.88(s, 2H, Ar-CH 2 ), 6.68(d, 2H, Ph-H), 6.81(t, 1H, Ph-H), 6.94(s, 1H, Ar-H), 7.14(t, 2H, Ph-H), 7.60(s, 1H, Ar-H); 13 C NMR: δ(ppm)3.43(CH 3 CH 2 ), 13.02 (CH 3 CH 2 ), 31.41 (CMe 3 ), 31.94 (CMe 3 ), 34.18 (CMe 3 ), 35.78 (CMe 3 ), 46.97, 47.86, 52.10, 54.41 (N(CH 2 CH 2 ) 2 N), 63.51 (Ar-CH 2 ), 116.43, 120.34, 125, 45, 127.12, 129.39, 138.96,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com