Alatrofloxacin parenteral compositions
A composition, gastrointestinal technology, applied in the field of 96/00756, nalididone antibiotic profloxacin mesylate, can solve problems such as insufficient removal of weak polar impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Batch Resin Processing of Profloxacin Mesylate
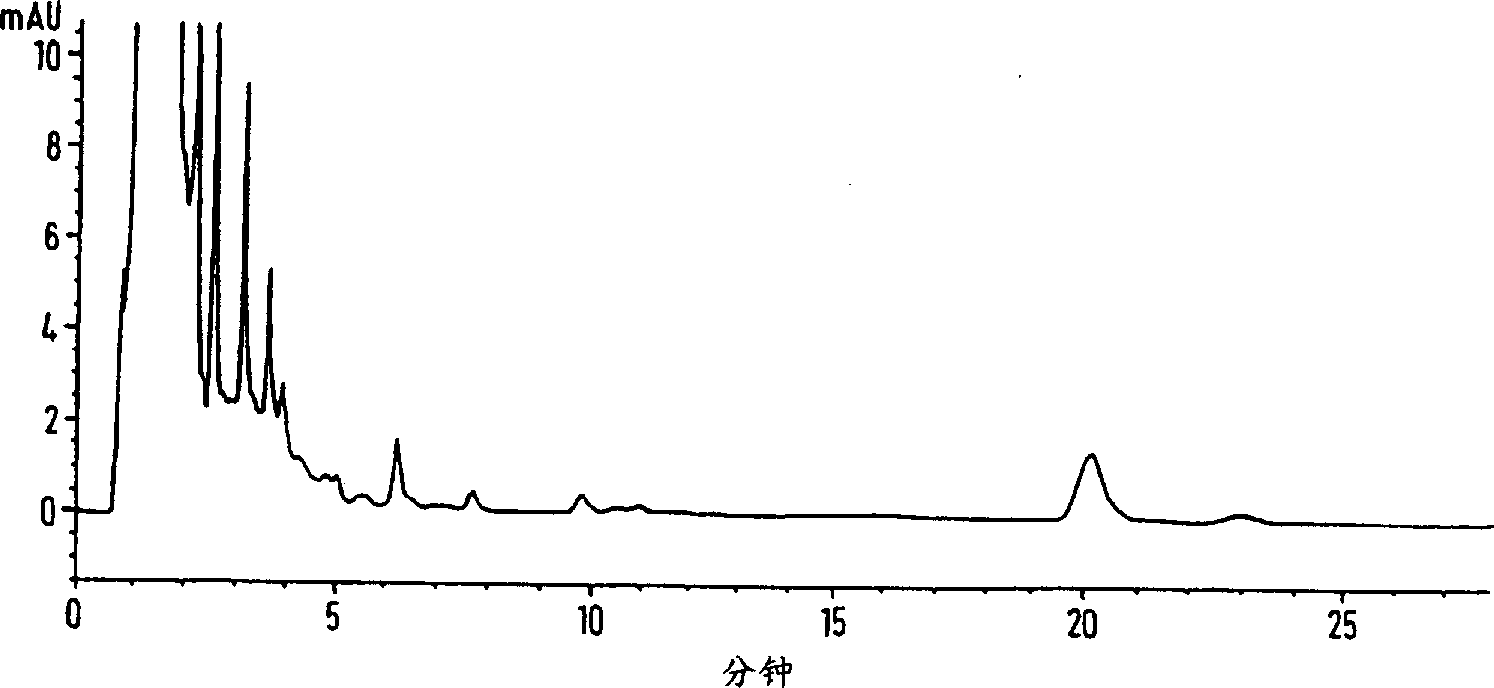
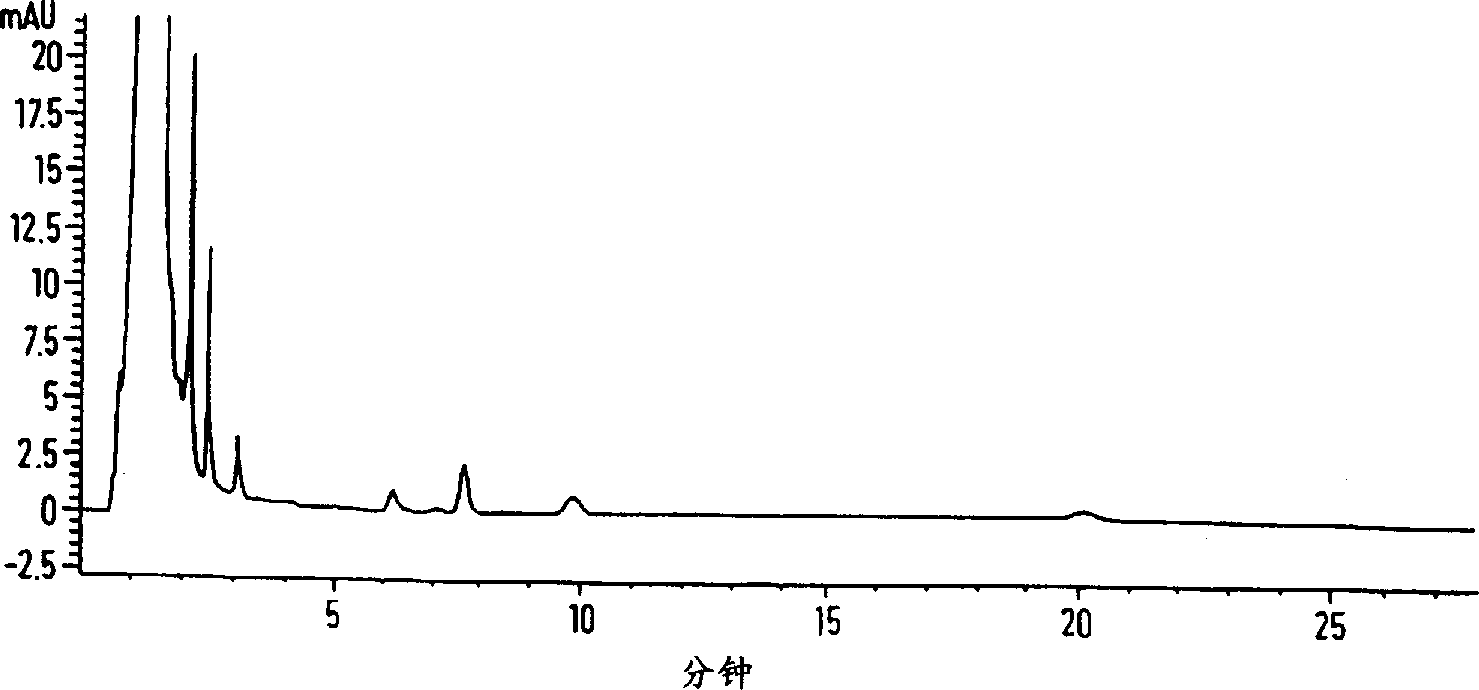
[0045] Profloxacin mesylate (50 g), which contained 700 ppm oligomer impurities in addition to other weakly polar impurities, was dissolved in 0.05% aqueous methanesulfonic acid (714 ml), and Mitsubishi Diacon HP-20® hydrophobic resin ( 50g). After stirring the resin suspension for 24 hours in the dark, the suspension was filtered and the solution was analyzed by high performance liquid chromatography (HPLC) (HPLC conditions as described above). The filtered solution contained 19 ppm of oligomeric impurities and the yield of profloxacin mesylate was 80%. The oligomer has the following NMR: ( 1NMR DMSO d
[0046] 6) δ: 9.98(d, 1H); 8.79(s, 1H); 8.51(s, 1H), 8.45(d, 1H), 8.16(d 1H), 8.03(d 1H), 7.92(d
[0047] 1H), 7.76(m 2H), 7.61(m 2H), 7.33(m 2H), 4.18(q 1H), 3.77(q 1H), 3.60(m 8H), 2.54(s 1H),
[0048] 2.34(s 1H), 2.27(s 3H), 1.92(b.s.2H), 1.72(b.s.2H), 1.28(d 3H), 1.17(d 3H).
Embodiment 2
[0050] Resin Column Treatment of Profloxacin Mesylate
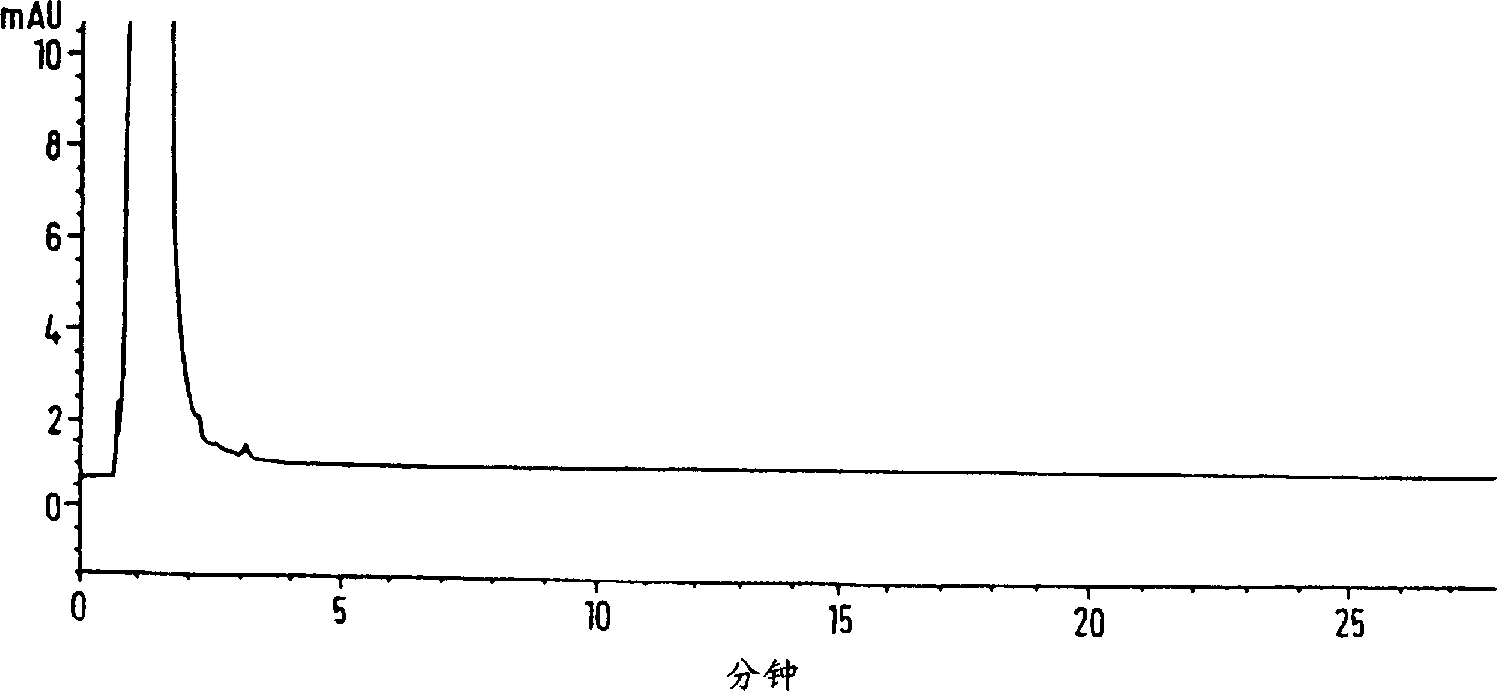
[0051] Profloxacin mesylate is dissolved in 10 to 40 liters of water per kilogram of drug substance at ambient temperature. The solution was filtered and then passed through a column containing hydrated Amberchrom CG-161(R) polystyrene resin (approximately 10-12 kg of crude reaction product per kg of dry resin) (3-5 bed volumes per hour). The column was washed with water (3 L / kg crude reaction product), then the eluate and wash were combined. The treated profloxacin mesylate solution was analyzed by HPLC. After being treated with a polystyrene resin column, the impurity content of formula II is reduced from 500ppm to below 30ppm, calculated as a drug substance. Additionally, the drug was recovered from the resin in 95% yield.
Embodiment 3
[0053] Separation of Zwitterions
[0054] Add 10% aqueous caustic agent dropwise to the solution containing profloxacin mesylate (10g) (prepared by embodiment 1 or 2), deionized water (154ml) and ethanol (31ml) that has been processed with resin, Neutralize to pH 6.5-7.5. After granulating the suspension for 30 minutes at 24°C, it was filtered. After the filtered solid was rinsed with ethanol (30ml), the zwitterions were dried under vacuum at 40°C for about 16 hours. Profloxacin (800 g) was recovered as white trapezoidal crystals with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com