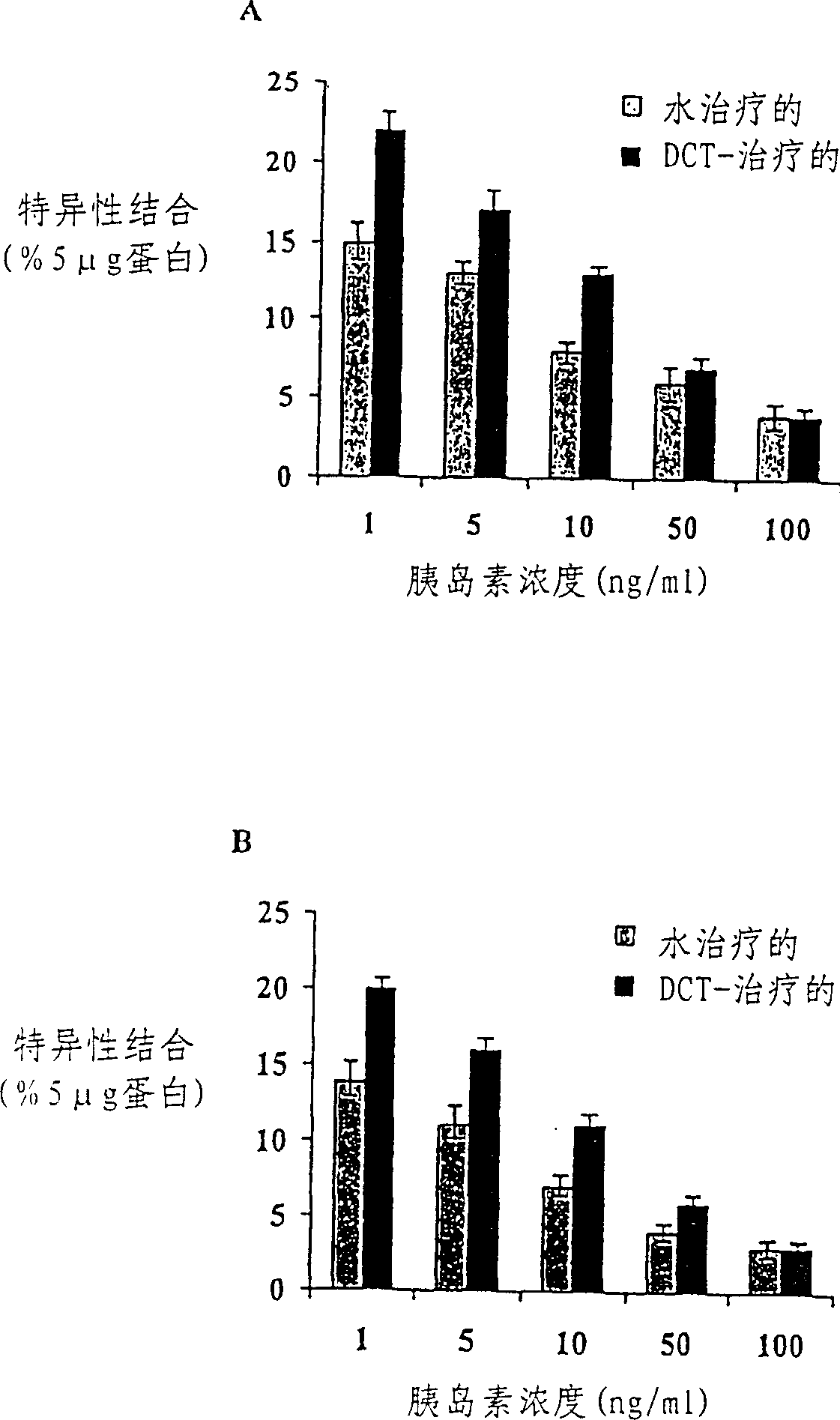
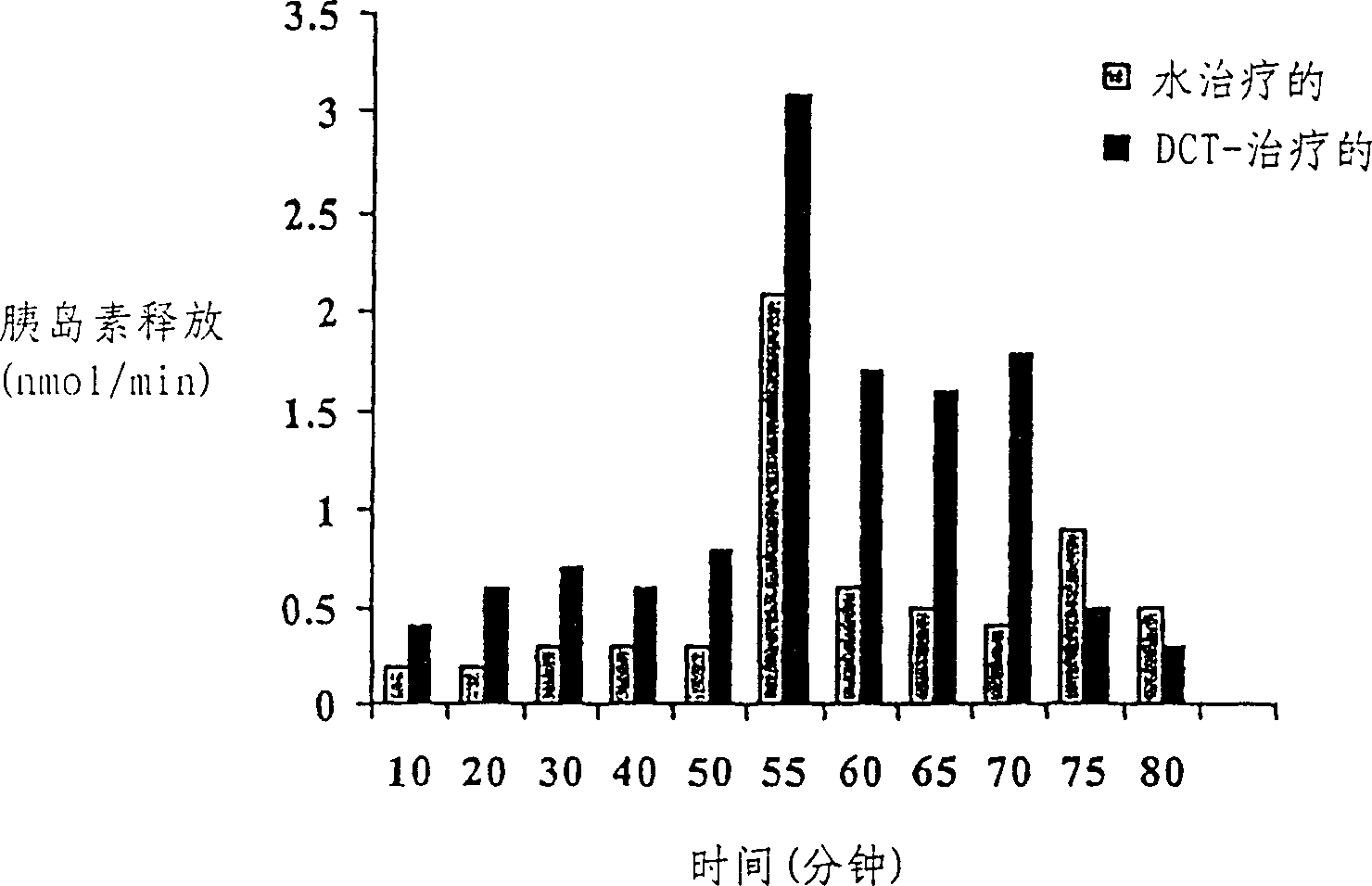
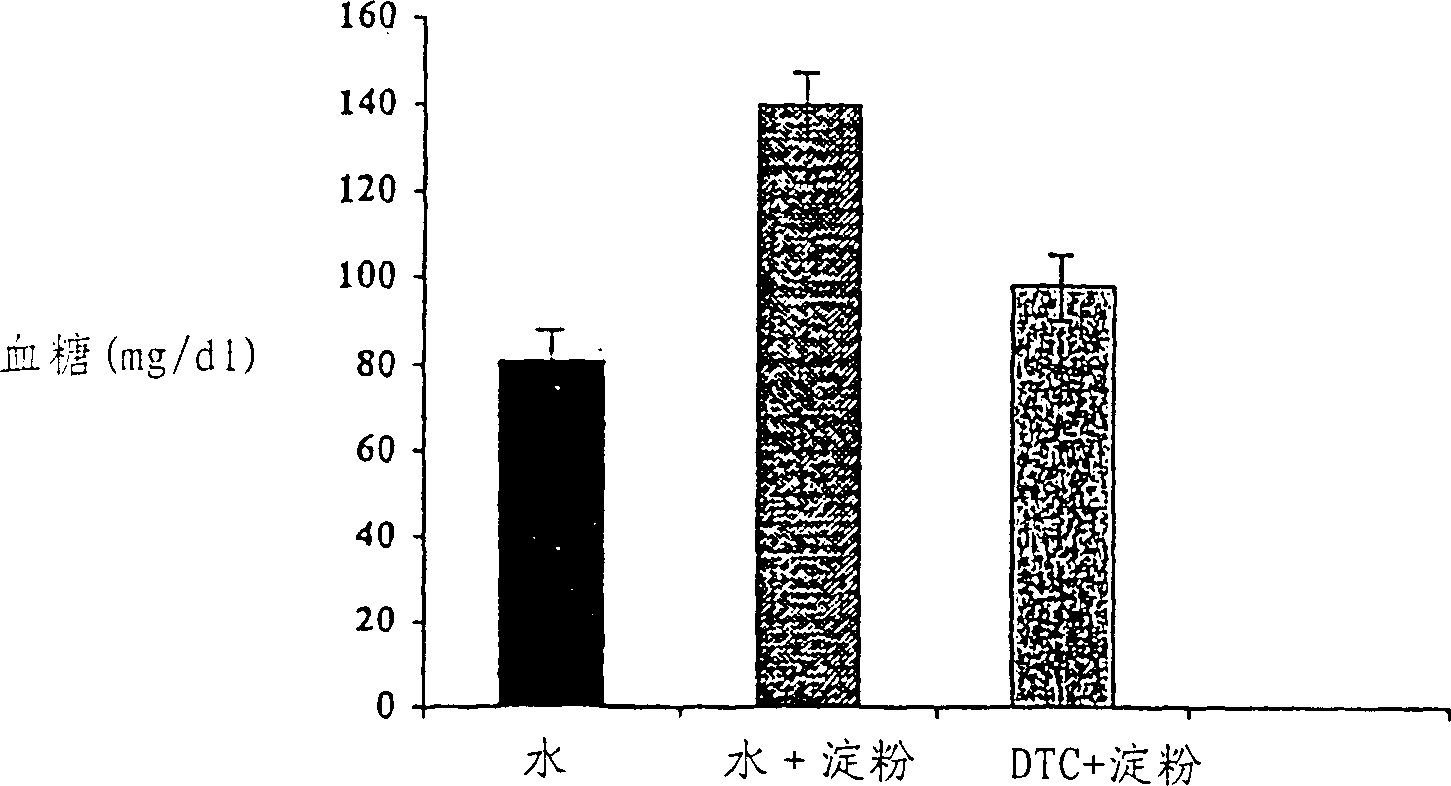
Midicial herbal compound for prevention and treatment of diabetes
A composition and hypoglycemic technology, applied in the herbal composition for the prevention or treatment of diabetes, in the field of prevention or treatment of diabetes, which can solve the problems of increasing cardiovascular mortality and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1 - Materials and methods - Extraction of medicinal plants
[0111] Flowering palm trees are grown in India; the inside of the trunk is collected and dried. Mulberry is grown in Korea and northern China; the leaves and roots are collected and dried. Ginseng is grown in Vietnam; the whole plant, including roots, branches, and leaves, is collected and dried. Ophiopogon japonicus, Rosa rugosa var typica (Regel) and Trichosanthes rugosa (Maxim) and Anemarrhena (Bunge) are grown in Korea and northern China; the roots are collected and dried. Commelina (L.) is grown in Korea and China; leaves, stems and flowers are collected and dried. Grind each dry ingredient into powder and mix (Flower Palm (about 20%), Ginseng (about 20%), Mulberry (about 15%), Ophiopogon japonicus (about 10%), Trichosanthes (about 10%), Anemarrhena (about 10%), Rose (about 8%) and Commelina (about 7%) w / w). Boil 40g of combined plant powder and 200ml of water in a slow cooker until the volume...
Embodiment 2
[0112] Example 2 - Goto-Kakizaki (GK) Rats
[0113] GK rats (Goto Y et al., Spontaneous diabetes produced by selective feeding of normal Wistar rats, Proc. Japan. Acad. 51:80-85, 1975; Sugiyama Y et al., Hypoglycemia produced in spontaneously diabetic GK rats The role of hepatic insulin sensitivity, J.Japan.Diab.Soc., 32:593-599, 1989) is one of the best animals to study NIDDM, because most of these animals develop diabetes at about 3 months of age, and diabetes Symptoms share many pathological features with human NIDDM. GK rats display impaired insulin secretion and peripheral insulin resistance. Insulin response, sensitivity to glycogen synthesis, lipogenesis, and DNA synthesis in hepatocytes of GK rats were significantly decreased compared with non-diabetic control rats. In GK rats, the islet architecture was disrupted, and areas of distinct fibrosis were seen in the stroma. As the disease progressed, beta-cell degranulation was observed, whereas lymphocytic infiltration...
Embodiment 3
[0114] Example 3 - Administration of MHCTD to GK Rats
[0115] In order to study the control of blood glucose by MHCTD in diabetic GK rats, 3-month-old rats were treated with the above-prepared MHCTD for 5 months. Animals were gavaged daily (10 am) with 2.5 ml (5 g / kg body weight). Age- and sex-matched GK rats were gavaged with an equal volume of water as controls. Blood glucose levels were measured weekly with a One Touch glucose meter until 1 year old.
[0116] To study the effect of MHCTD on the prevention of diabetes, GK rats were treated with MHCTD from 3 weeks of age before the onset of NIDDM. Rats were gavaged daily with MHCTD (5 g / kg body weight) or water as described above until 13 weeks of age and blood glucose levels were measured weekly until 13 weeks of age.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com