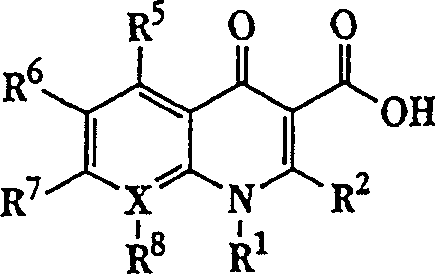
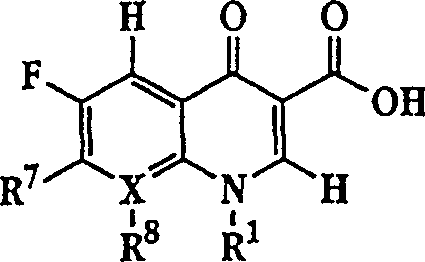
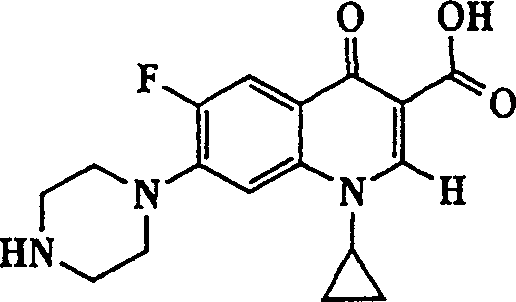
Quinolone-containing liposome composition
A composition and liposome technology, applied in the directions of liposome delivery, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., can solve the problem of high leakage rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0131] Preparation of Glycyl-Ciprofloxacin and Lysyl-Ciprofloxacin
[0132] A. Synthesis of Glycyl-Ciprofloxacin and Lysyl-Ciprofloxacin
[0133] N-TBOC protected amino acids are commercially available. The compound was reacted with an equimolar amount of N-hydroxysuccinamide and a 10% excess of dicyclohexylcarbodiimide (DCC) in dichloromethane to form the N-hydroxysuccinamide ester.
[0134] The ester is reacted with 2 moles of triethylamine and 1 mole of anhydrous carboxy-protected ciprofloxacin HCl in anhydrous solvent to form the ciprofloxacin amide of the TBOC-protected amino acid.
[0135] Ciprofloxacin-amide was deprotected with trifluoroacetic acid and isolated by methods appropriate to the physical and chemical properties of the product. For example, L-threonine-ciprofloxacin is recovered by adjusting the pH of the aqueous suspension to 7 to separate the crystalline conjugate. The L-leucine-ciprofloxacin conjugate was recovered by extraction of the pH 7-8 aqueous ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com