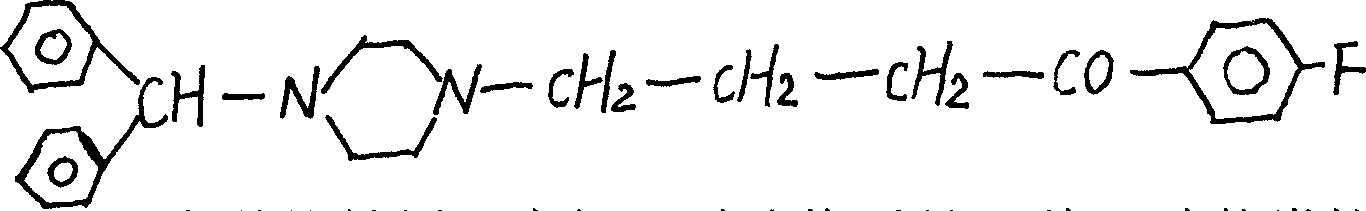Process for preparing bis-phenyl fluorozine
A technology of difluzine and benzhydrylpiperazine, which is applied in the field of preparation of difluzine, can solve problems such as heart damage and cerebrovascular stealing, and achieve the effects of increasing yield, simplifying process, and qualified quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: The best preparation method of difenfluzine, including the following steps:
[0014] 1) Prepare by reacting 104 grams of γ-butyrolactone with 108 grams of thionyl chloride in the presence of 4 grams of anhydrous zinc chloride as a catalyst to obtain 114 grams of γ-chlorobutyryl chloride (boiling point 85° / 8666Pa), Reverse copy and take; 2) Then mix an appropriate amount of solvent 840ml of dichloromethane, 134g of fluorobenzene, 196.8g of aluminum chloride, cool to below 8℃, add 196.8γ-chlorobutyryl chloride dropwise, and stir at 8-10℃ 1 hour, stirring at room temperature for 6 hours, standing overnight, ice-decomposition, the water layer was extracted with 3*150ml dichloromethane, dried with calcium chloride, and then dichloromethane was distilled off, collected by distillation under reduced pressure at 156-158℃ / With the 4533Pa fraction, 248 grams of γ-chloro-4-fluorobenzene-1-butanone was obtained. 3) Use 63 g of benzyl alcohol to react with 300 ml of concentr...
Embodiment 2
[0015] Example 2-Calcium antagonistic properties: using classic and most suitable rabbit aortic ring specimens for studying calcium antagonists, using high potassium (KCL) to depolarize the open voltage-dependent calcium channel, using norepinephrine (NE ) Open receptor-gated calcium channels and observe the effect of dipfluzine on these two channels. It was found that the relaxation effect of dipfluzine on vasoconstriction caused by KCL and NE is similar to cinnarizine in nature, but its blocking voltage-dependent calcium channel effect is 4 times stronger than that of cinnarizine.
Embodiment 3
[0016] Example 3-Selective expansion of blood vessels: The isolated porcine arterial ring experiment was used to compare the effects of difenfluzine on the contraction of the isolated porcine basilar artery, coronary and radial arteries caused by KCL. It was found that difenfluzine inhibits KCL. Isolated porcine basilar artery, coronary artery and radial artery contracted PD 2 Respectively 5.7+0.6 (n=6), 5.4+_0.4 (n=6) and 4.6+_0.5 (n=5), that is, the selectivity to the basilar artery is the highest, and it is significantly stronger than cinnarizine ( PD 2 Is 5.0+_0.4 n=6 p<0.55).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com