Peptide as antagonist of VEGF receptor Flt-1
An antagonist, flt-1 technology, applied in the direction of receptors/cell surface antigens/cell surface determinants, peptides, specific peptides, etc., can solve the problem of unsatisfactory angiogenesis inhibitors, weak systemic toxicity and anti-angiogenesis effect And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
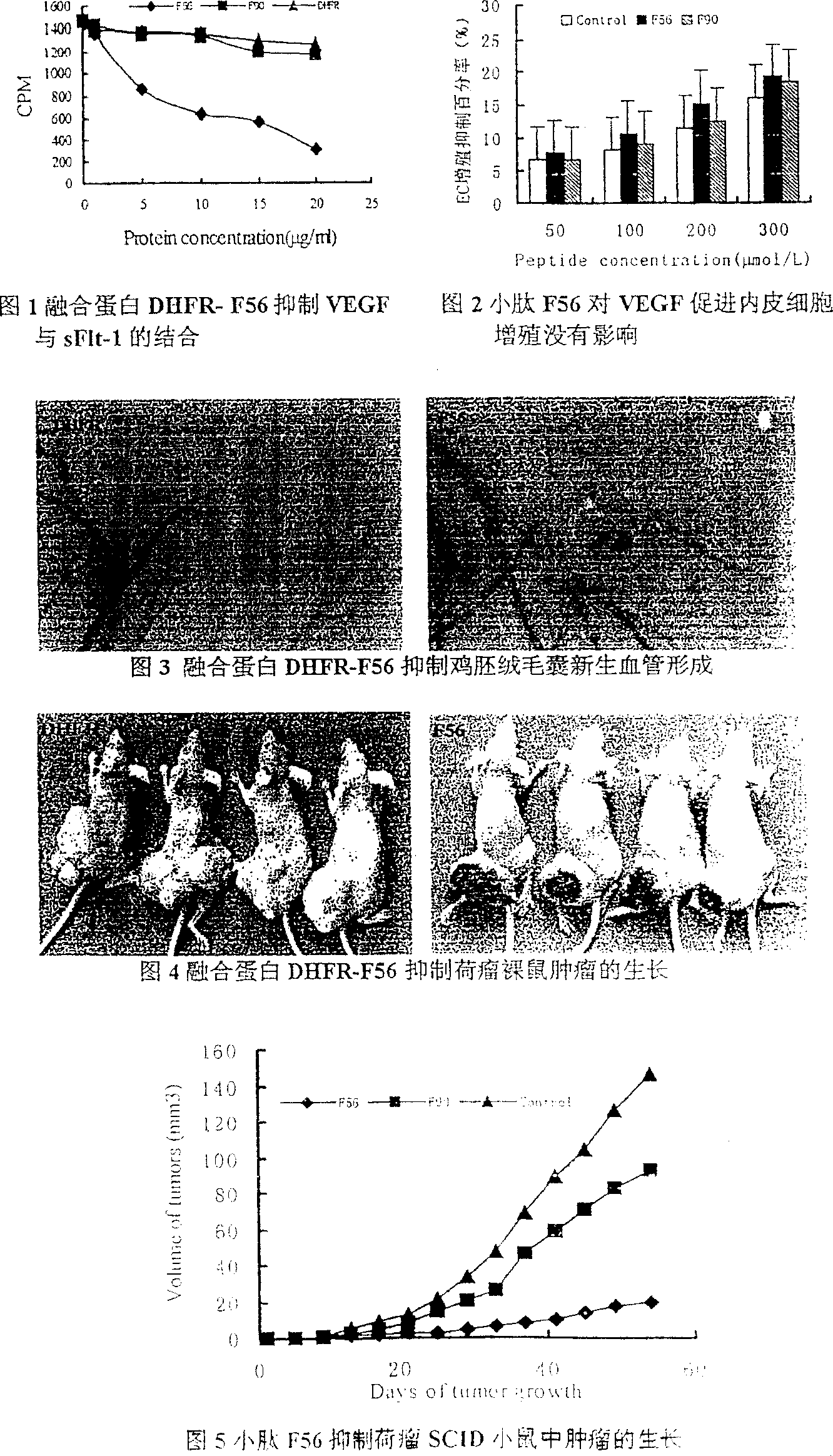
Image
Examples
Embodiment 1
[0097] h 2 N-Trp-His-Ser-Asp-Met-Glu-Trp-Trp-Tyr-Leu-Leu-Gly-COOH (F56)
[0098] Put the peptide synthesis column packed with 4-(2',4'-dimethoxyphenyl-Fmoc-aminomethyl)phenoxyacetamidoethyl resin in the PioneerTM peptide synthesizer, and in nitrogen atmosphere in the following order: The peptide synthesis was carried out as follows:
[0099] 1. Solvate the resin in DMF for 5 minutes;
[0100] 2. Treat the resin with 20% piperidine in DMF for about 15 minutes to remove the protecting group Fmoc on the resin grafted group (or on the resin-bound amino acid α-amino group);
[0101] 3. Wash the resin with DMF for about 1 minute;
[0102] 4. Activate the first amino acid Gly at the C-terminus with a 0.2M solution of HBTU and HOBT in DMSO-NMP (N-methylpyrrolidone) and a 0.4M solution of diisopropylethylamine in DMSO-NMP (Fmoc-Gly ) of the α-carboxyl group;
[0103] 5. Coupling the activated amino acid obtained in step 4 with the resin (or resin-bound amino acid) of step 2 in DMF...
Embodiment 2
[0110] h 2 N-Trp-His-Val-Asp-Glu-Thr-Trp-Trp-Leu-Leu-Met-Leu-COOH (F87)
[0111] The title peptide was prepared according to the procedure described in Example 1. The first amino acid is Fmoc-Leu, and the following amino acids are added sequentially according to the conditions: Fmoc-Met→Fmoc-Leu→Fmoc-Leu→Fmoc-Trp→Fmoc-Trp→Fmoc-Thr(tBu)→Fmoc-Glu(γ -OtBu)→Fmoc-Asp(β-OtBu)→Fmoc-Val→Fmoc-His(Trt)→Fmoc-Trp. This gave 35 mg of the title peptide.
Embodiment 3
[0113] h 2 N-Trp-His-Asp-Pro-Thr-Pro-Trp-Trp-Ser-Trp-Glu-Ile-COOH (F90)
[0114] The title peptide was prepared according to the procedure described in Example 1. The first amino acid is Fmoc-Ile, and the following amino acids are added sequentially according to the conditions: Fmoc-Glu(γ-OtBu)→Fmoc-Trp→Fmoc-Ser(tBu)→Fmoc-Trp→Fmoc-Trp→Fmoc-Pro→ Fmoc-Thr(tBu)→Fmoc-Pro→Fmoc-Asp(β-OtBu)→Fmoc-His(Trt)→Fmoc-Trp. 44 mg of the title peptide were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com