Production process of pyrrolidone carboxylic acid and its salt
A technology of pyrrolidone carboxylic acid and production method, applied in directions such as organic chemistry, can solve problems such as not being effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Production of pyrrolidone carboxylic acid from L-glutamic acid
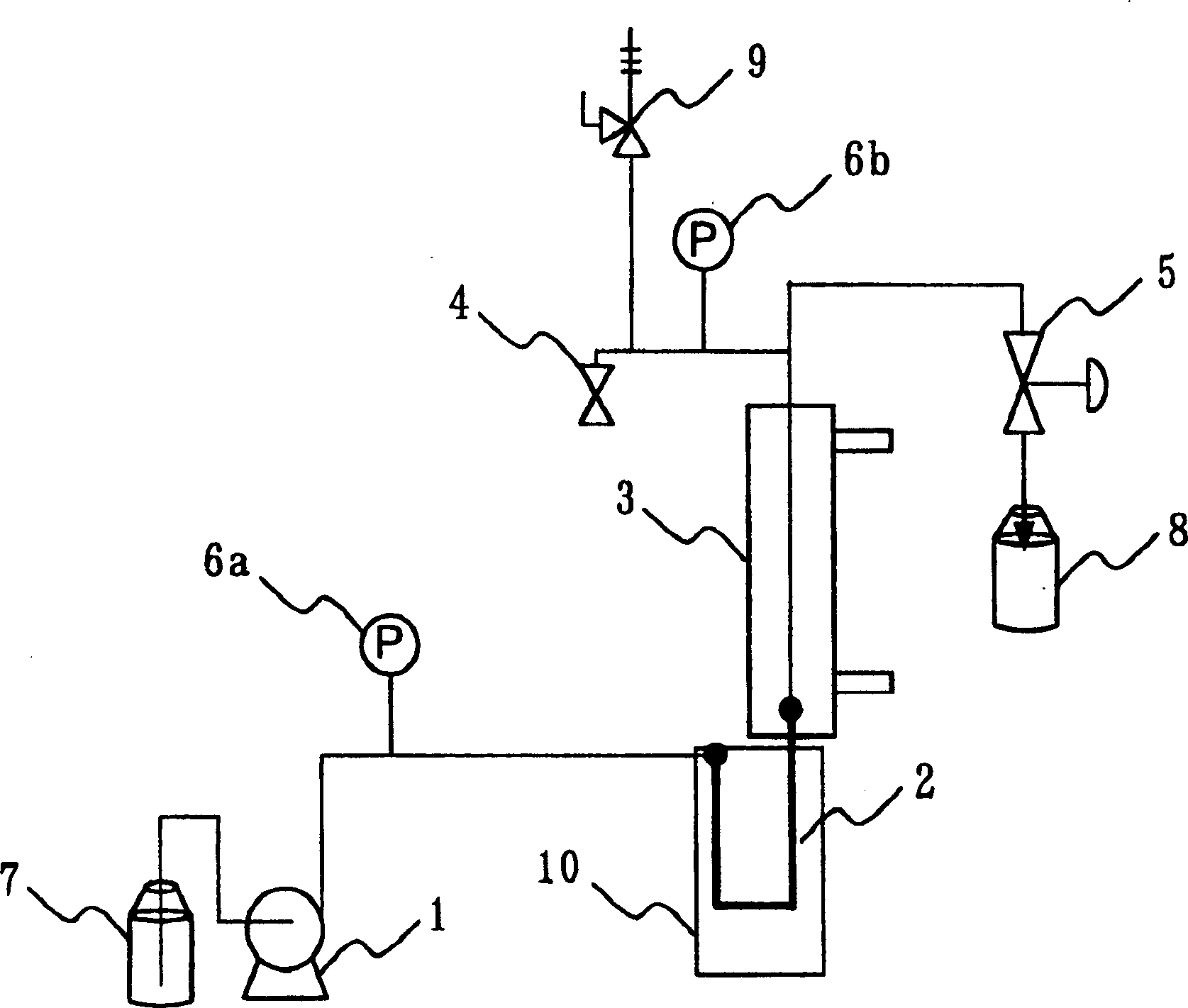
[0033] Water was added to L-glutamic acid to make a 33 wt% dispersion. use figure 1equipment, the L-glutamic acid slurry was fed by a high-pressure pump (1) to a reactor (2) (stainless steel, cylindrical, inner diameter 3.87 mm) heated to 200° C. to start the reaction. The residence time, or time through the reactor (2), is controlled to be 100 seconds when water flows alone. The reaction is terminated by introducing a jacketed heat exchanger (3) equipped at the outlet of said reactor (2), which is rapidly cooled. The pressure is adjusted through the check valve (5) in the system, thereby maintaining the pressure from the output end of the high-pressure pump (1) to the check valve (5) at 2 MPa.
[0034] After evaporation of the water, the prepared reaction mixture was recovered as a solid. The produced pyrrolidone carboxylic acid and unreacted glutamic acid contained in the solid were rediss...
Embodiment 2
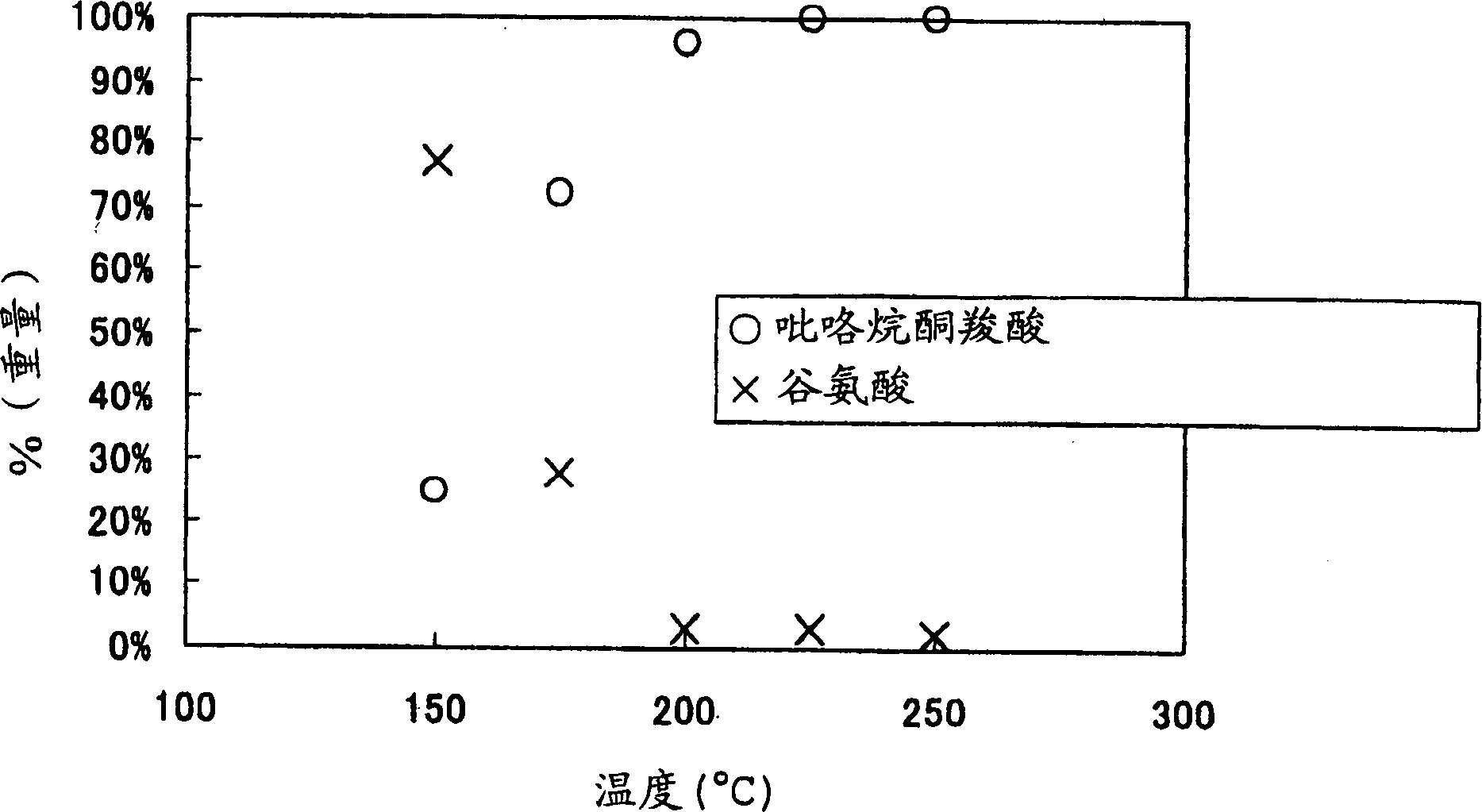
[0037] Embodiment 2: Reaction temperature dependence
[0038] The dispersion (33wt%) of L-glutamic acid was reacted in the same manner as in Example 1, except that the temperature was changed to 150°C-250°C, the pressure was changed to 5MPa, and the residence time was controlled to be 100 seconds when water alone flowed .
[0039] In the same manner as in Example 1, water was evaporated from the prepared reaction mixture to recover a solid, which was redissolved and analyzed by the HPLC method to quantitatively determine pyrrolidonecarboxylic acid and glutamic acid. The results are shown in figure 2 .
Embodiment 3
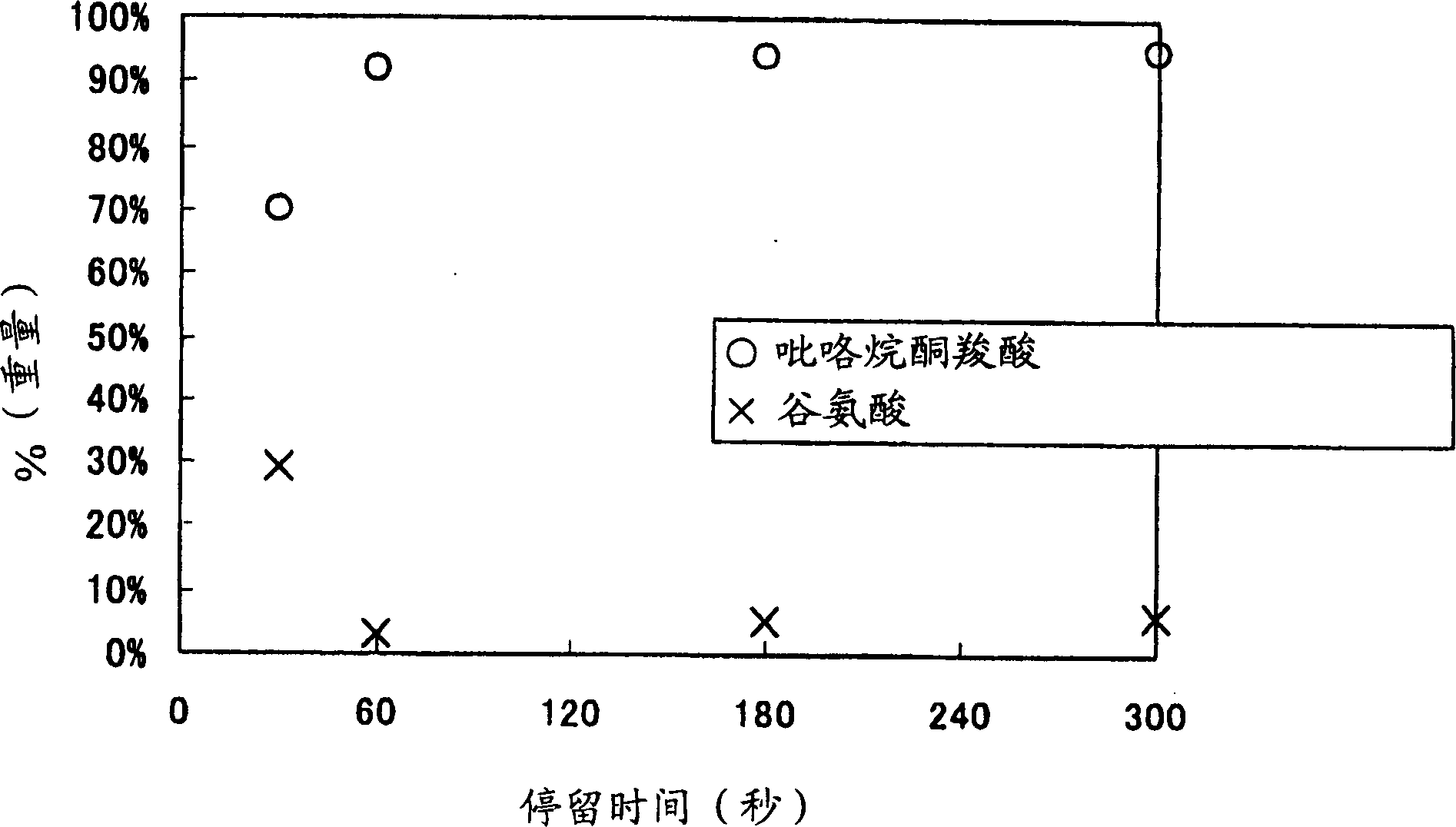
[0040] Example 3: Residence time dependence
[0041] A dispersion of L-glutamic acid (33 wt%) was reacted in the same manner as in Example 1, except that the temperature was changed to 200° C., the pressure was changed to 5 MPa, and the residence time was controlled to be 30-300 seconds when water alone flowed.
[0042] In the same manner as in Example 1, water was evaporated from the prepared reaction mixture to recover a solid, which was redissolved and analyzed by the HPLC method to quantitatively determine pyrrolidonecarboxylic acid and glutamic acid. The results are shown in image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com