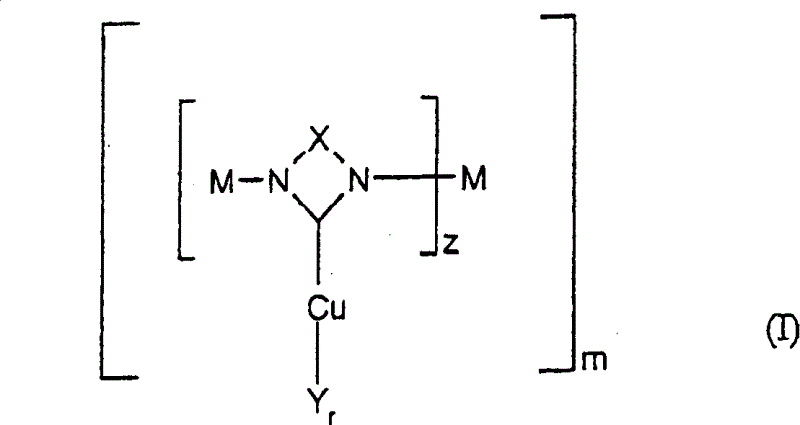
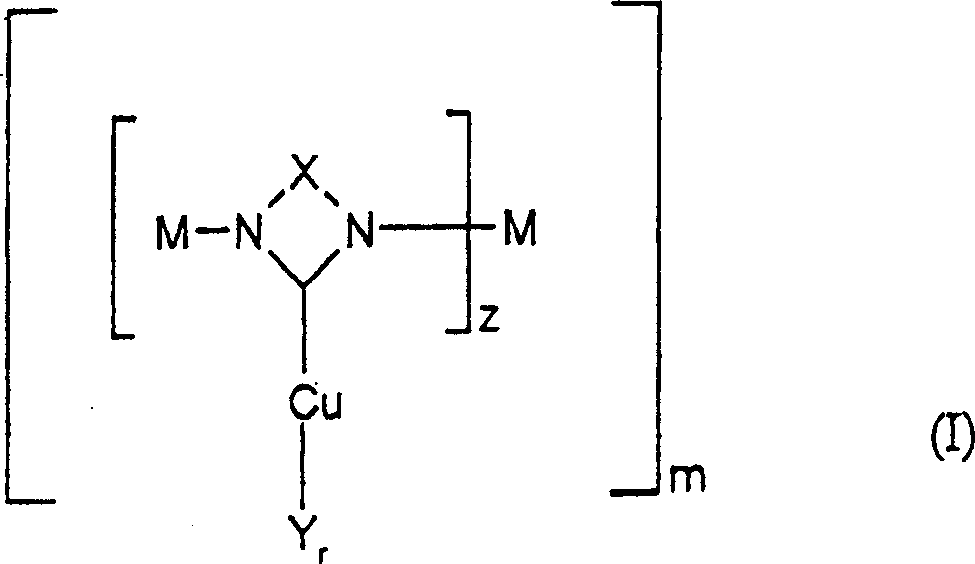
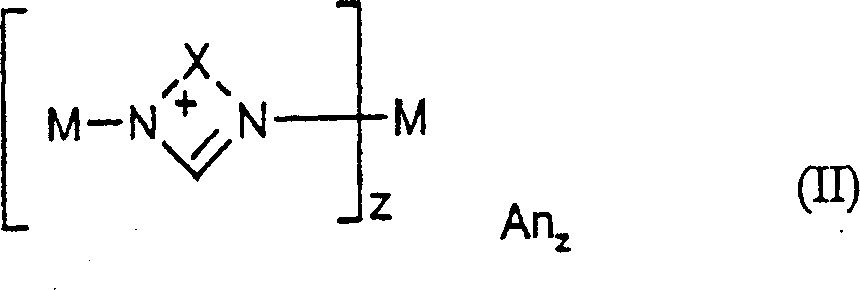Method for preparing nitrodiphenylamine
A technology of nitrodiphenylamine and nitrochlorobenzene, which is applied to the preparation of amino compounds from amines, organic chemical methods, preparation of amino compounds, etc., and can solve the problems of selectivity reduction and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] {1,3-Di-[N-(N'-methyl)imidazolylene-methyl]-5-methylbenzene}-copper(II) bromide, C 17 h 20 Br 2 CuN 4 (x2KBr)
[0072]
[0073]Diimidazolium salt (135 mg, 0.31 mmol) was dissolved in 10 ml of toluene under argon, and potassium tert-butoxide (71 mg, 0.63 mmol) was added at 0°C. After 2h, copper(II) bromide (70mg, 0.31mmol) was added and the mixture was stirred for 12h. The solvent was then removed under vacuum to give the product as an off-white powder.
[0074] FD / MS: 343(M-2Br, main component), 423(M-Br), 503(M+2H)
Embodiment 2
[0076] Preparation of {1,3-di-[N-(N'-methyl)imidazolylene-methyl]-benzene}-copper(II) bromide, C 15 h 17 Br 2 CuN 5 (x2KBr)
[0077]
[0078] Diimidazolium salt (131 mg, 0.31 mmol) was dissolved in 10 ml of toluene under argon, and potassium tert-butoxide (71 mg, 0.63 mmol) was added at 0°C. After 2h, copper(II) bromide (70mg, 0.31mmol) was added and the mixture was stirred for 12h. The solvent was then removed under vacuum to give the product as an off-white powder.
[0079] FD / MS: 330(M-2Br, main component), 410(M-Br), 490(M+2H)
Embodiment 3
[0081] Preparation of [N,N′-bis(2-pyridyl)imidazolylene]-copper(II) bromide, C 13 h 10 Br 2 CuN 4 (xKBr)
[0082]
[0083] Diimidazolium salt (92 mg, 0.31 mmol) was dissolved in 10 ml of toluene under argon, and potassium tert-butoxide (36 mg, 0.31 mmol) was added at 0°C. After 2h, copper(II) bromide (70mg, 0.31mmol) was added and the mixture was stirred for 12h. The solvent was then removed under vacuum to give the product as an off-white powder.
[0084] FD / MS: 364 (M-Br, main component).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com