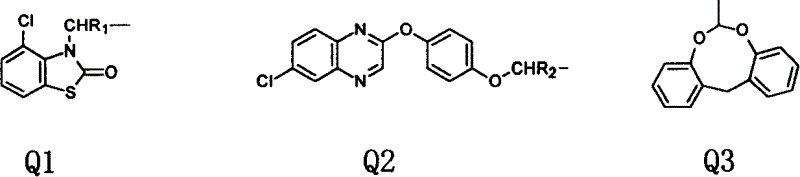
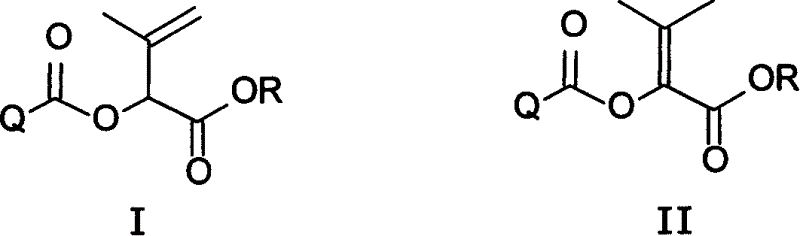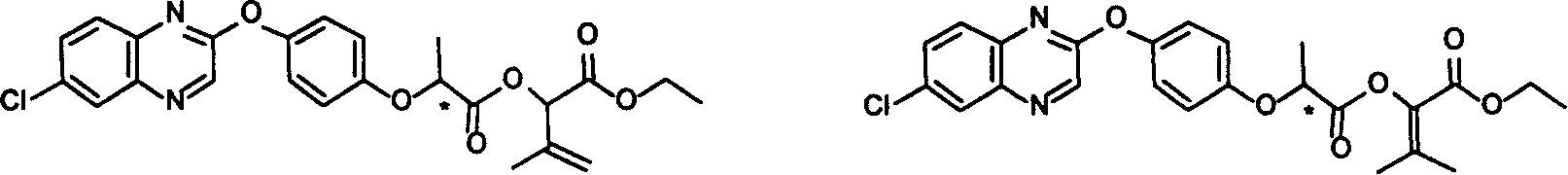Compound of alpha, beta and beta, gamma unsaturation carboxylate class of possessing weeding activity
An ester compound, unsaturated technology, applied in organic chemistry, animal repellent, plant growth regulator, etc., can solve the problems that have not been disclosed, and achieve the effect of broad-spectrum herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0051] Synthesis of Compound 2:
[0052]
[0053] Add A (R type, 30.0 grams, 0.0804 moles, available from the market) and 80 milliliters of tetrahydrofuran (THF) in a 500 milliliter reaction flask, stir until fully dissolved, add a solution of 3.2 grams of sodium hydroxide and 80 milliliters of water, and stir at room temperature for 5 After 1 hour, the reaction was stopped, and 100 ml of ethyl acetate was added for extraction. The aqueous phase was acidified with concentrated hydrochloric acid to obtain the intermediate acid as a white solid (B1), which weighed 25 g after drying.
[0054]
[0055] Add B1 (20.0 g, 0.058 mol) and 150 ml of dichloromethane into a 250 ml reaction flask, stir, add oxalyl chloride (11.0 g, 0.087 mol), 3 drops of DMF, stir at room temperature for 4 hours, concentrate to obtain 23 g of acid chloride , the appearance is a yellow liquid (C1).
[0056]
[0057] Add A1 (0.68 gram, 3.3 mmol) in the reaction bottle of 50 milliliters, triethylamin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com