Topical composition containing at least one vitamin D or one vitamin D analogue and at least one corticosteroid
A corticosteroid, composition technology, applied in the field of topical composition, can solve the problem of non-compliance with the treatment system and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Gel formulation containing calcipotriol and betamethasone
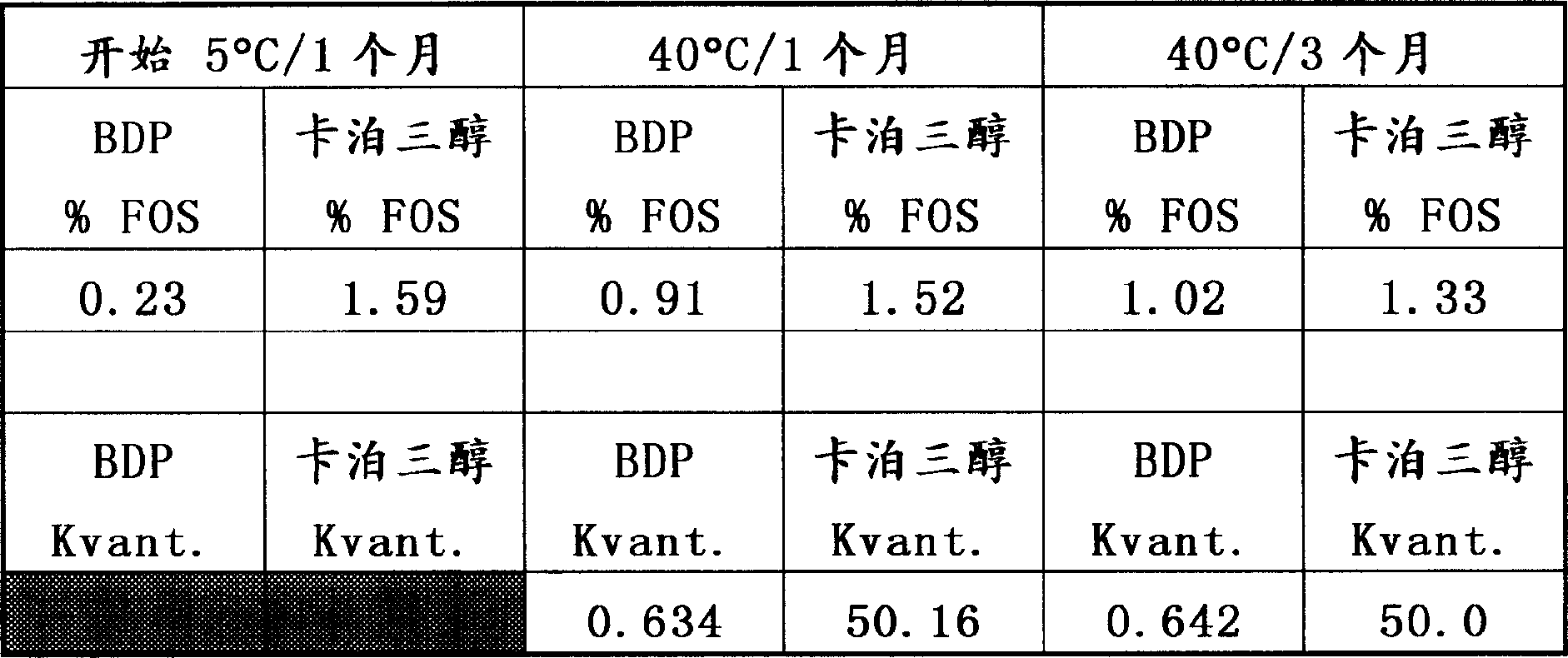
[0140] To prepare 1 kg of gel formulation, 30 g of hydrogenated castor oil were melted together with 749 g of heptamethylnonane at 85-90°C and cooled to about 60°C with homogenization. The mixture was then cooled to 25-30°C with stirring. 643 mg of betamethasone dipropionate suspended in 50 g of heptamethylnonane was added to the homogenized gel matrix. Dissolve 52.2 mg of calcipotriol hydrate or 50 mg of calcipotriol in 170 g of polyoxypropylene-15-stearyl ether and add to the mixture of other ingredients to homogenize the preparation to ensure uniform distribution of the active ingredient. The resulting gel formulation was stable when stored at 40°C for 3 months, indicating a shelf life of at least 2 years at room temperature. Stability data are shown in Tables 1 and 2 below.
[0141] Table 1
[0142]
[0143] Start at 5°C / 1 month
40℃...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com