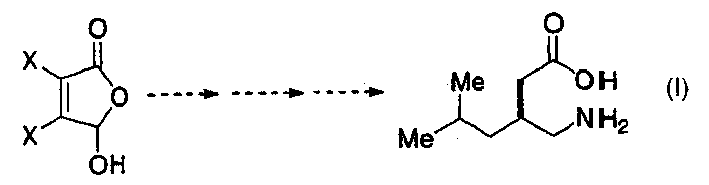Process for preparing highly functionalized gamma-butyrolactams and gamma-amino acids
An aryl and alkyl technology, which is used in the field of preparation of highly functionalized γ-butyrolactam and γ-amino acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
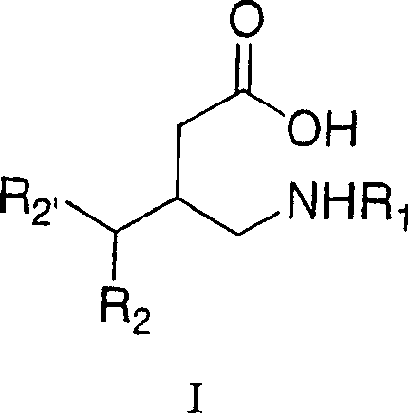
[0100] The inventive process for preparing 3-substituted gamma-aminobutyric acids disclosed herein has a number of advantages. First, they obtain 3-substituted γ-aminobutyric acids such as furgabalin or its analogues such as 3-aminomethyl-5-methyl-octanoic acid with a minimum of steps and under mild conditions. Second, they use generally inexpensive and readily available reagents. Third, they exploited the synthetic possibility of viscohaloacids.
[0101] Mucic acid 1 (2,3-dichloro-4-oxo-2-butenoic acid) and mucbromic acid (2,3-dibromo-4-oxo-2-butenoic acid) are commercially available and Inexpensive starting material. Both molecules are characterized by a carbon-carbon double bond with a Z configuration, two halogen atoms and two carbonyl groups. This high degree of functionality makes both mucchloric acid and mucbromic acid particularly useful as building blocks for the synthesis of various biologically active heterocycles: such as substituted 1,5-dihydropyrrol-2-ones, py...
Embodiment
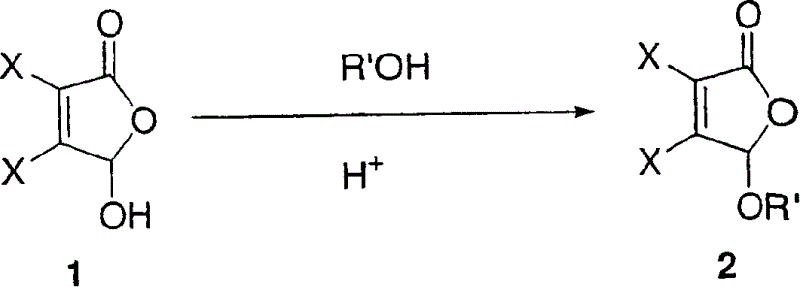
[0201] Route A, Option 2
[0202] Step A: 5-Benzyloxy-3,4-dihalo-5H-furan-2-one
[0203] In 1000 mL of toluene and in an apparatus equipped with a Dean-Stark trap (strap), mix viscohalic acid (0.4-0.6 mol, 1 eq), benzyl alcohol (1.5 eq), and p-toluenesulfonic acid (0.05 eq) . The mixture was heated at reflux until water collection in the Dean Stark trap ceased. The mixture was then cooled to room temperature. The toluene was removed in vacuo at 35-40 °C to leave the crude product as a very light amber oil. The crude product was purified by silica gel column chromatography eluting with 55% then 10% ethyl acetate in heptane.
[0204] 1. 5-Benzyloxy-3,4-dichloro-5H-furan-2-one. Prepared as provided in Procedure A. 95% yield. 1 HNMR (CDCl 3 , 300MHz) δ7.3(s, 5H), 5.92(s, 1H), 4.95(d, 1H), 4.89(d, 1H). C 10 h 8 Cl 2 o 3 The elemental analysis measured value (theoretical value): C, 51.12 (50.99); H, 2.93 (3.11); N, <0.05 (0.00); Cl, 27.19 (27.37).
[0205] 2. 5-Benzyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com