Combinations of (a) an ATP-competitive inhibitor of c-acl kinase activity with (b) two or more other antineoplastic agents
A competitive inhibitor and kinase activity technology, applied in antineoplastic drugs, drug combinations, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0070] In the following preferred embodiments of the invention, more general terms may be replaced independently or entirely by the more specific definitions given above, thus resulting in more preferred embodiments of the invention.
[0071] Preferred combinations of the invention comprise (a) N-{5-[4-(4-methyl-piperazin-1-yl-methyl)-benzamido]-2-methylphenyl}-4 -(3-pyridyl)-2-pyrimidine-amine, or a pharmaceutically acceptable salt thereof, and (b) at least two other antineoplastic agents, independently in free form or in pharmaceutically acceptable salt form, preferably the above defined antineoplastic agents.
[0072] More preferred combinations of the present invention comprise (a) N-{5-[4-(4-methyl-piperazin-1-yl-methyl)-benzamido]-2-methylphenyl}- 4-(3-pyridyl)-2-pyrimidine-amine, or a pharmaceutically acceptable salt thereof, and (b) two or three, preferably two, selected from purine nucleoside analogs and topoisomerase II inhibitors Other antineoplastic agents can be...
Embodiment 1
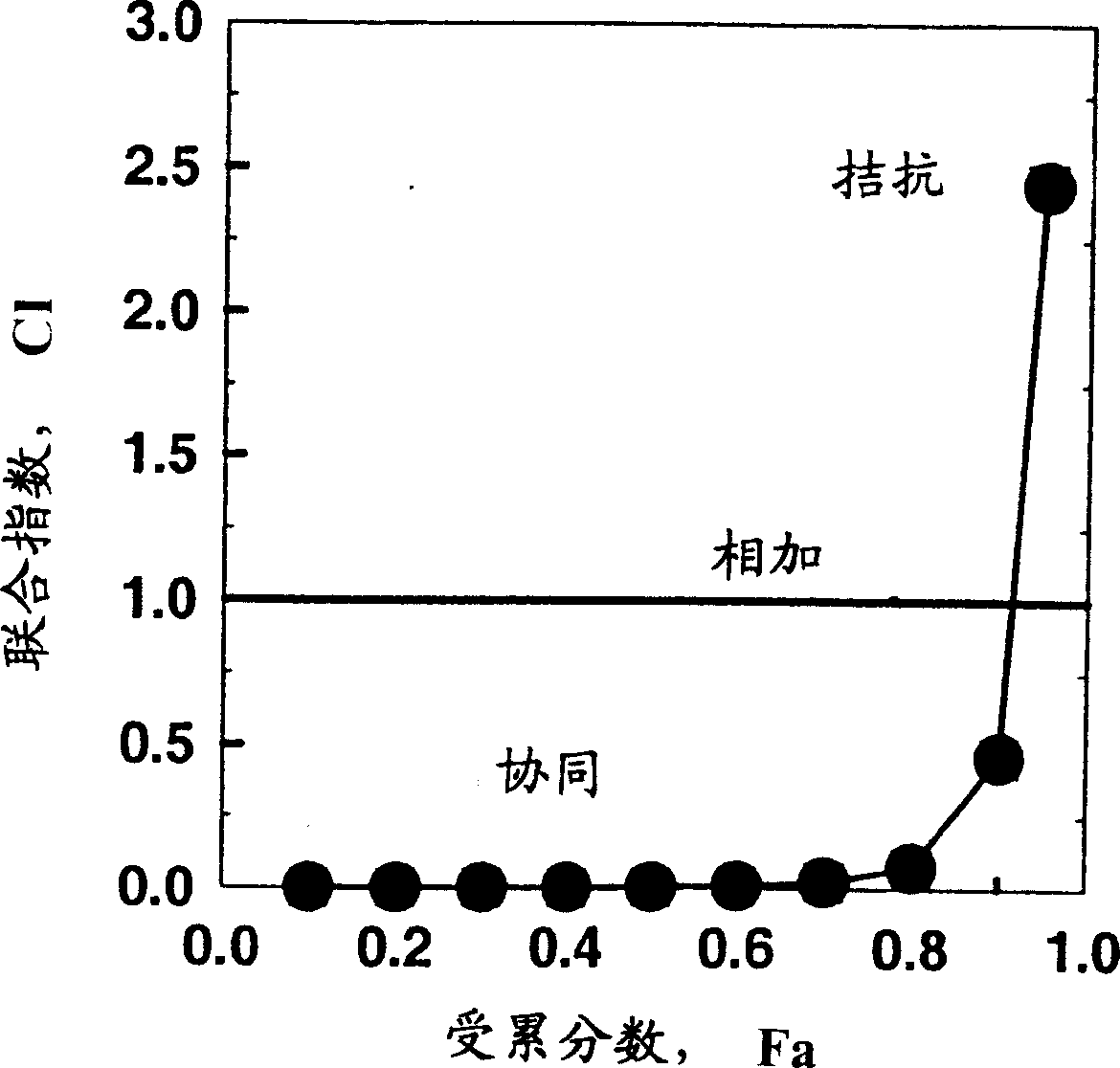
[0128] Example 1: N-{5-[4-(4-methyl-piperazin-1-yl-methyl)-benzamido]-2-methylbenzene Base}-4-(3-pyridyl)-2-pyrimidine-amine monomethanesulfonate (STI571) with fludarabine and cytarabine (ara-C) combination - effect on CEM / 0 cells
[0129] effective dose
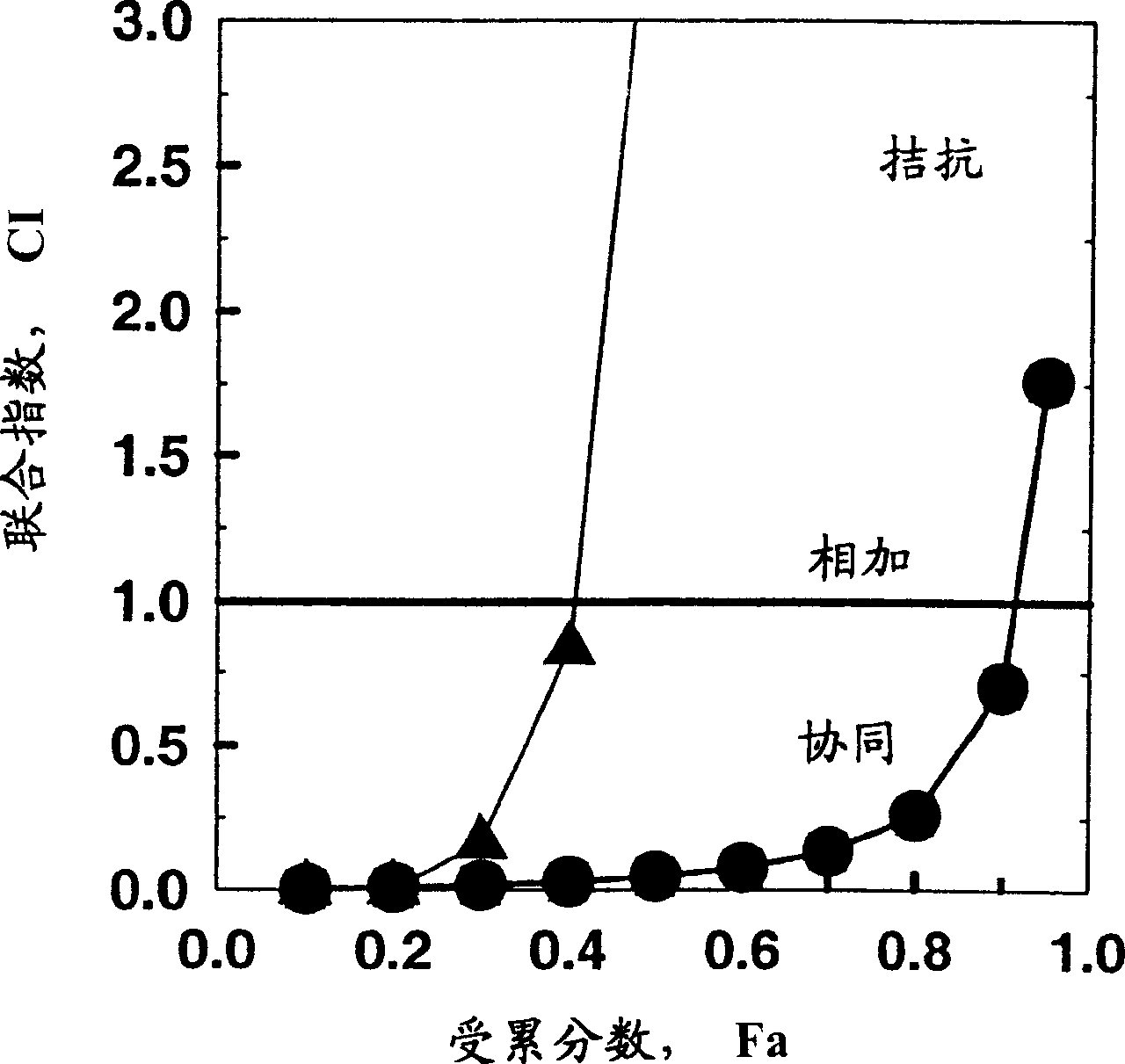
[0130] From these data it can be seen that the ED 50 and ED 70 Synergy found between STI571 and fludarabine and ara-C, but in ED 90 No synergistic effect was found.
Embodiment 2
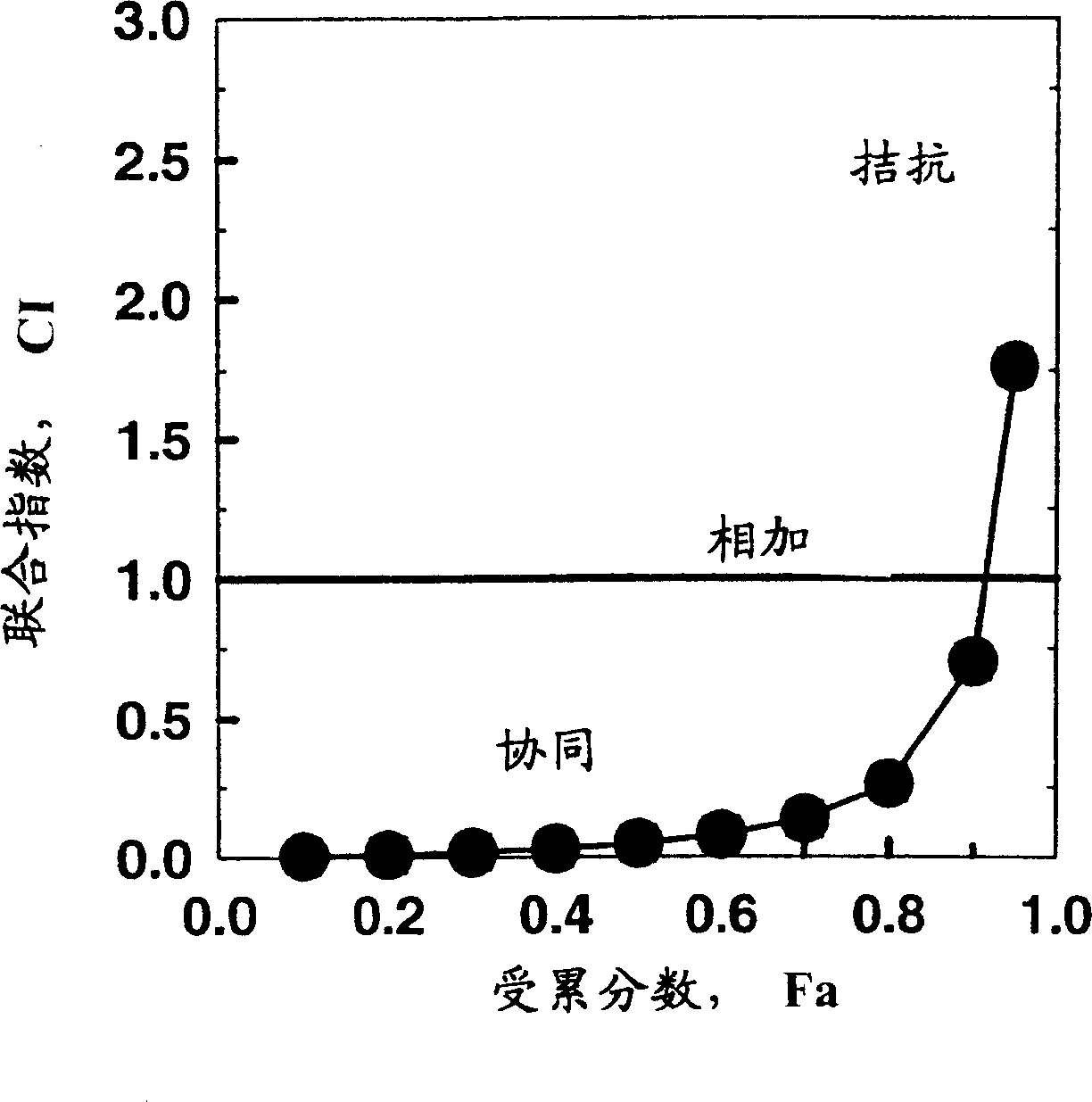
[0131] Example 2: N-{5-[4-(4-methyl-piperazin-1-yl-methyl)-benzamido]-2-methylbenzene Base}-4-(3-pyridyl)-2-pyrimidine-amine monomethanesulfonate (STI571) with fludarabine and cytarabine (ara-C) Combination with fludarabine first - effect on CEM / 0 cells
[0132] effective dose
[0133] Visible: Compared with Example 1, in ED 50 and ED 70 When fludarabine treatment was preceded by STI treatment for 4 hours, a smaller drug synergy was found, and in the ED 90 However, no synergistic effect was found.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com