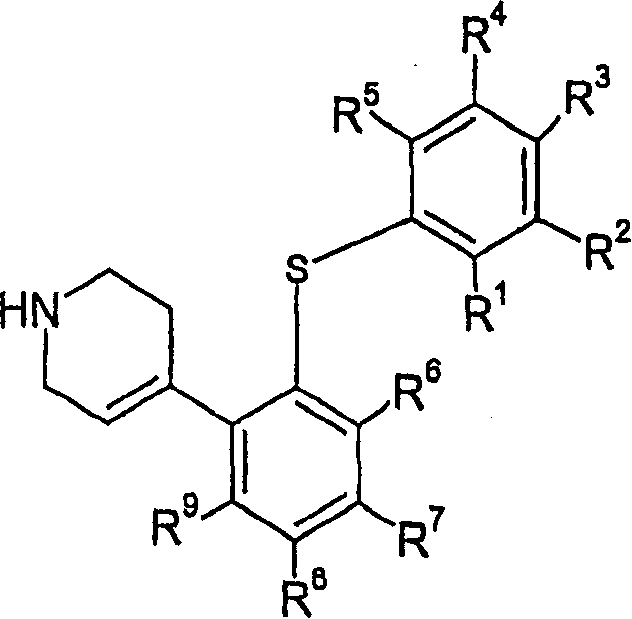
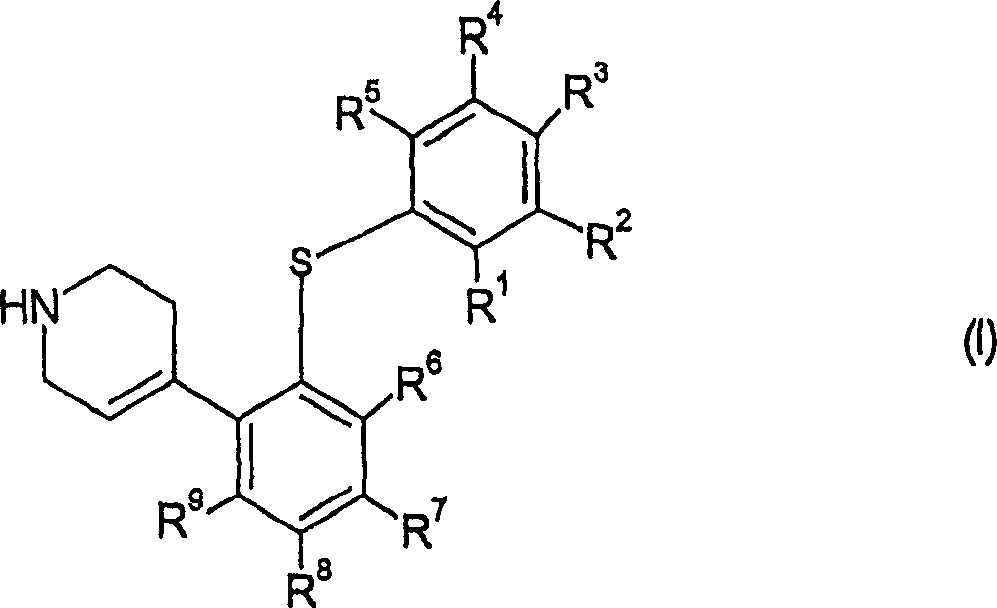
4-(2-phenylsulfanyl-phenyl)-1,2,3,6-tetrahydropyridine derivatives as serotonin reuptake inhibitors
A thio- and hydroxyl-based technology is applied in the field of 4-(2-phenylthio-phenyl)-1,2,3,6-tetrahydropyridine derivatives as a serotonin reuptake inhibitor, and can solve the problem of Drug therapy can not wait for the problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0316] Synthetic method A:
[0317] Compound 2: 4-[2-(4-Chlorophenylthio)-phenyl]-1,2,3,6-tetrahydropyridine
[0318] Concentrated aqueous hydrochloric acid (10 mL) was added to a stirred solution of 1-tert-butoxycarbonyl-4-[2-(4-chlorophenylthio)phenyl]piperidin-4-ol (0.84 g, 2 mmol) in acetic acid ( 30mL). The solution was boiled at reflux overnight, cooled to room temperature, then stirred in an ice bath. Aqueous NaOH (9.1 M, 40 mL) was slowly added and the cloudy solution was extracted with ethyl acetate (2 x 40 mL). The combined organic phases were dried (MgSO4) and the solvent was evaporated in vacuo. The crude product (0.48 g) was dissolved in ethyl acetate (3.2 mL) at 50 °C, and a solution of oxalic acid (0.11 g) in EtOH (3.2 mL) was added slowly. Collect the white oxalate salt of the target compound. 1 H(DMSO-d 6 )δ7.3-7.2(m, 7H); 7.15(m, 1H); 7.00(m, 1H); 5.6(d, 1H); 3.7(d, 2H); 3.25(t, 2H); , 2H); LC / MS (m / z) 302.1 (MH + ); RT = 2.29; Purity (UV, ELSD): 100%...
Embodiment 2
[0320] Synthetic method B:
[0321] Compound 24: 4-[2-(2-Chlorophenylthio)-phenyl]-1,2,3,6-tetrahydropyridine
[0322] Bis[(2-diphenylphosphino)phenyl]ether (22mg; 0.04mmol) and bis(dibenzylidene)palladium (23mg; 0.04mmol) were dissolved in 2.5mL of toluene and added to 4-hydroxy -4-(2-Mercapto-phenyl)-piperidine-1-carboxylic acid tert-butyl ester (200 mg; 0.64 mmol) and 1-chloro-2-iodobenzene (200 mg; 0.84 mmol) in 4 mL of toluene. Potassium tert-butoxide (80 mg; 0.68 mmol) was added and the reaction mixture was stirred at 100° C. under argon for 16 hours. The reaction mixture was filtered through silica gel, eluting with ethyl acetate. Solvent was removed in vacuo. The residue was dissolved in 4 mL of glacial acetic acid and 1 mL of concentrated HCl. The mixture was stirred at 100°C for 16 hours. The solution was poured onto ice and 8 mL of 28% aqueous NaOH was added. The base suspension was extracted with ethyl acetate (2 x 50 mL). The combined organic phases were wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com