Activation and inhibition of the immune system
An inhibitor, encoded technology, applied in the field of activation and suppression of the immune system, can solve the problem that activation is not beneficial
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0199] Materials and methods
[0200] 1. Reagents
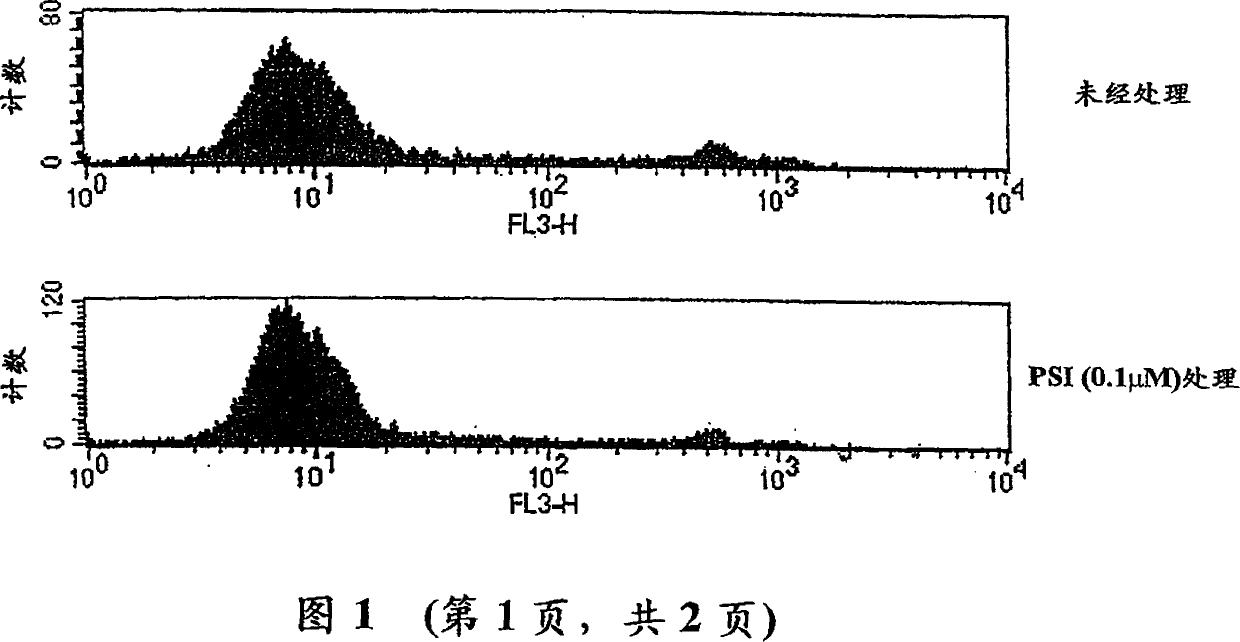
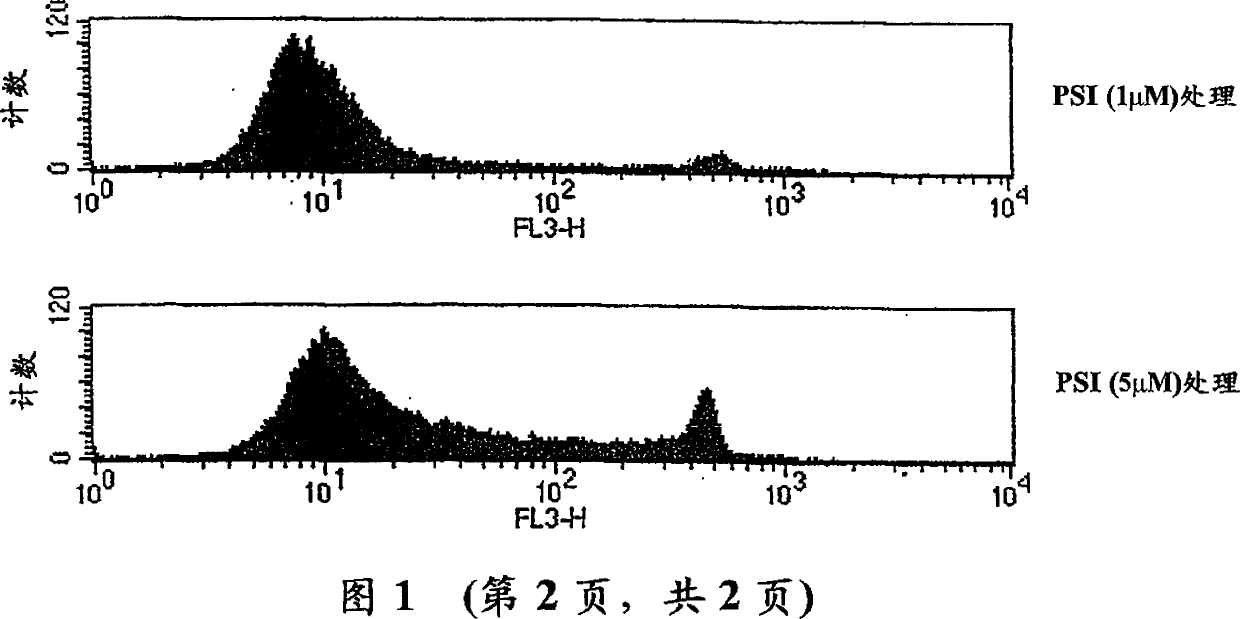
[0201] Human recombinant GM-CSF and TNFα were kindly provided by Dr. Glenn Larsen (GI) and Dr. D Tracey (BASF), respectively. Human recombinant IL-4 was purchased from R&D System (Minneapolis, USA). PMA, LPS and ionomycin were obtained from Sigma Chemical Co. (St Louis, USA). Proteosome inhibitor Cbz-Ile-Glu (O-tert-butyl-Ala-cerema (PSI) was obtained from Calbiochem (Nottingham, UK). M-CSF was obtained from Genetics Institute (Boston, USA).
[0202] 2. Preparation of Peripheral Blood Mononuclear Cells
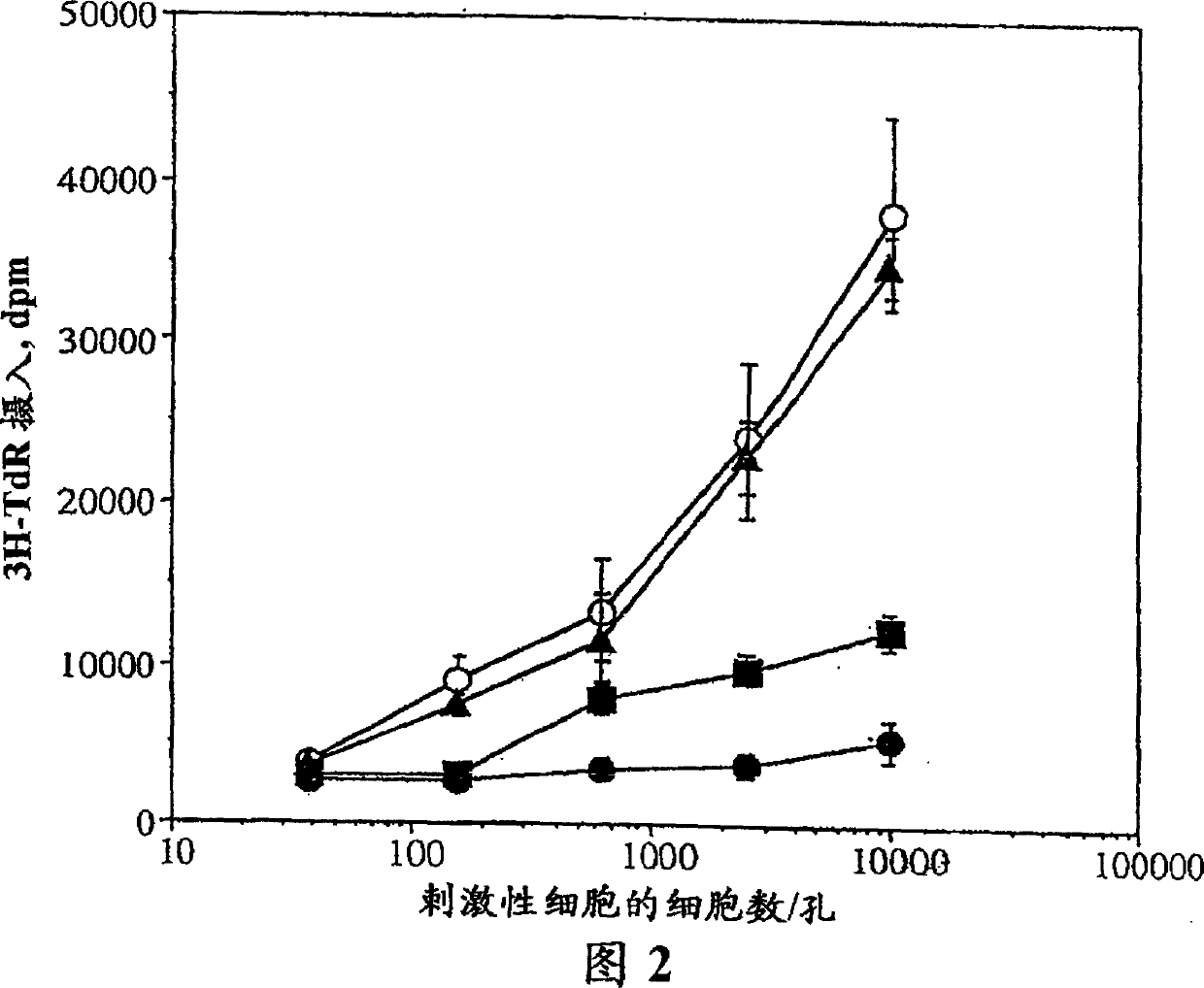
[0203] Peripheral blood mononuclear cells (PBMC) were obtained by density centrifugation of leukopheresis residues obtained from healthy volunteers (North London Blood Trahsfusion Service, Colindale, UK). The heparinized residue was diluted 2-fold with HBSS, 25 ml was carefully overlaid on an equal volume of Ficoll-Hypaque lymphoprep (Nycomed, Oslo, Norway) in a 50 ml sterile tube, and then centrifuged at 2000 rpm for 30 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com