Application of compound PSI in preparation of medicine for treating leucocythemia
A compound, leukemia technology, applied in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., can solve the problems of treatment failure, easy recurrence, and median survival time of less than 2 years
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
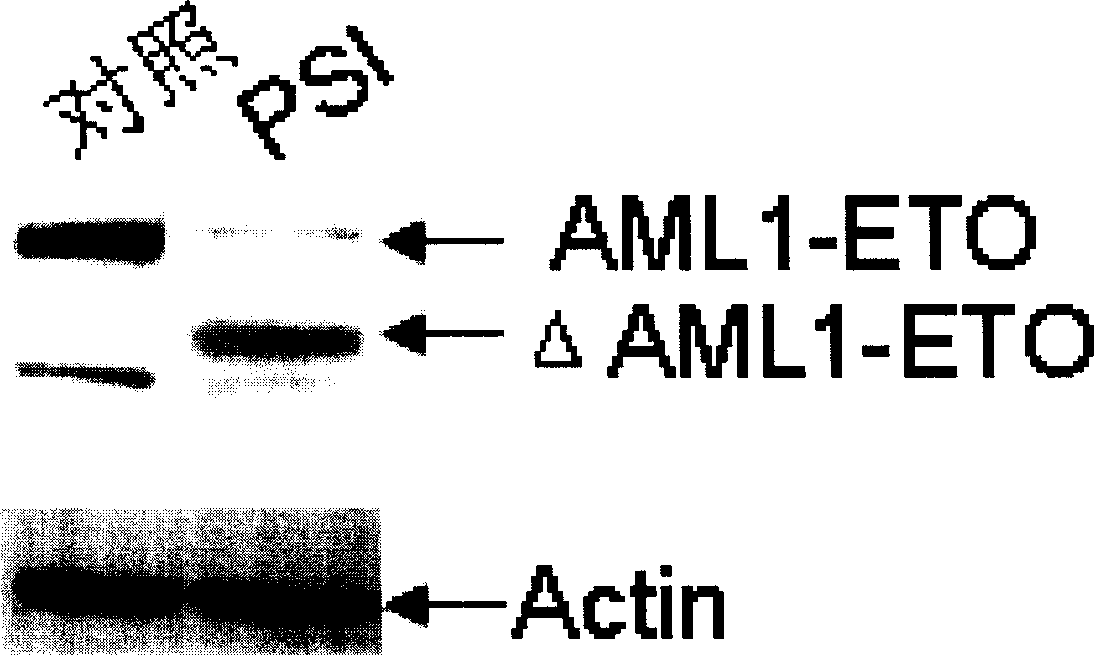
[0032] Example 1: Degradation effect of PSI on AML1-ETO fusion protein.
[0033] Kasumi-1 cells were treated with different concentrations of PSI, and after 24 hours of treatment, the cells were collected and lysed with 1× cell lysate to extract protein, and then Western blot was performed with anti-ETO antibody (purchased from Santa Cruz, USA). The results showed that PSI could regulate the expression of AML1-ETO fusion protein, degrade part of AML1-ETO protein and produce degradation bands. (See figure 1 , the left is the control, and the right is the change of AML1-ETO fusion protein after being treated with 50nM concentration of PSI for 24 hours. )
Embodiment 2
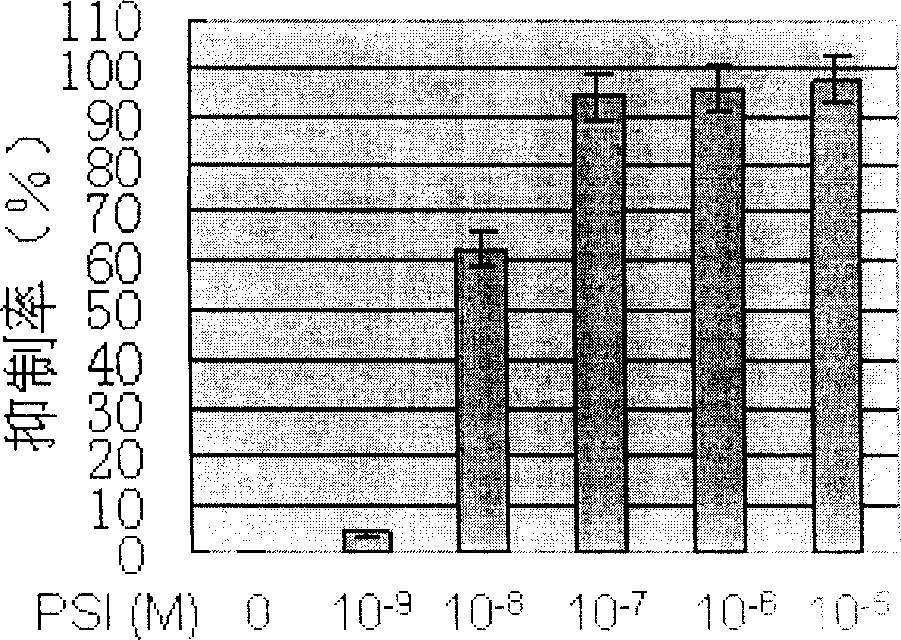
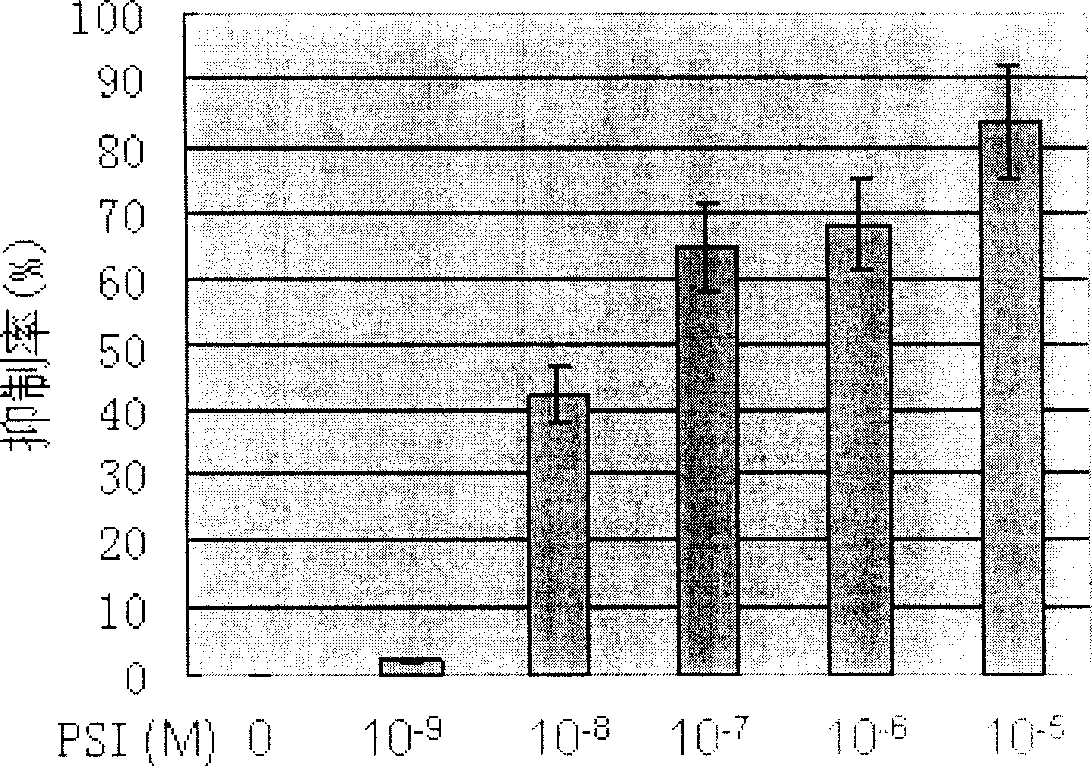
[0035] With different concentrations of 1×10 -9 ~1×10 -5 The PSI of M treated the cell line Kasumi-1 cells containing t(8; 21) chromosomal translocation and the CML cell line K562 cells containing t(9; 22) chromosomal translocation, and added 2-(2-formazan) after 44 hours CCK- 8 detection reagents, and then incubated for 4 hours, and then measured the absorbance (OD 450 ). It was found that PSI treatment could significantly reduce the OD of Kasumi-1 cells and K562 cells 450 , indicating that PSI can significantly inhibit the proliferation of Kasumi-1 cells and K562 cells, and the inhibition rate is positively correlated with the drug concentration (see figure 2 and image 3 ).
[0036] According to the growth inhibitory effect of PSI on Kasumi-1 cells, the half inhibitory concentration (IC) of PSI to Kasumi-1 cells was calculated. 50 ) is 3.84×10 -8 M.
[0037] According to the growth inhibitory effect of PSI on K562 cells, the half inhibitory concentration (IC) of P...
Embodiment 3
[0039] With different concentrations (1×10 -9 ~1×10 -6 M) PSI treatment of cell line Kasumi-1 cells containing t(8; 21) chromosomal translocation and CML cell line K562 cells containing t(9; 22) chromosomal translocation, respectively at 12, 24, 36, 48 hours after treatment Hours counted the number of living cells by fetal pan blue exclusion method and drew the growth curves of Kasumi-1 cells and K562 cells. It was found that PSI could significantly inhibit the growth of Kasumi-1 cells and K562 cells, and this effect was time-dose dependent. When the concentration of PSI is 10nM~100nM, the number of viable cells of cell line Kasumi-1 containing t(8;21) chromosomal translocation significantly decreased after PSI treatment, see Figure 4 . When the concentration of PSI is 20nM~160nM, the number of living cells of CML cell line K562 containing t(9;22) chromosome translocation significantly decreased after PSI treatment, see Figure 5 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com