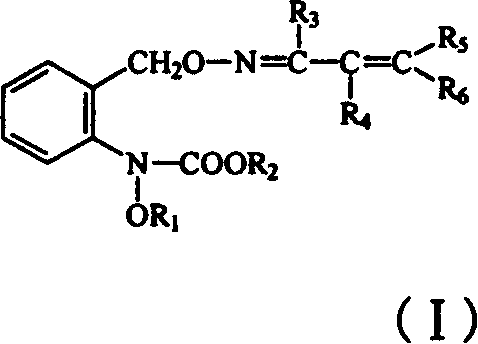
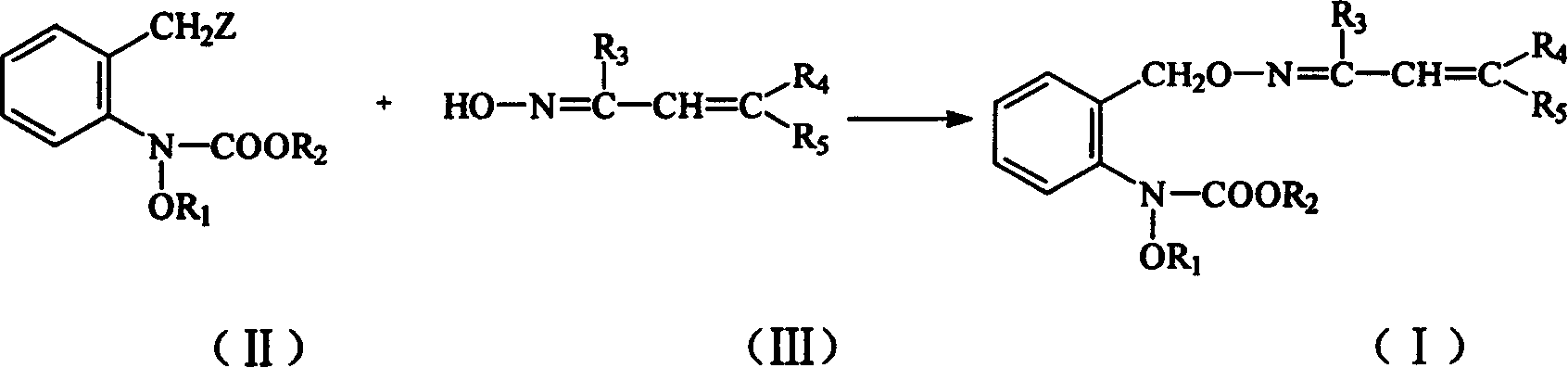
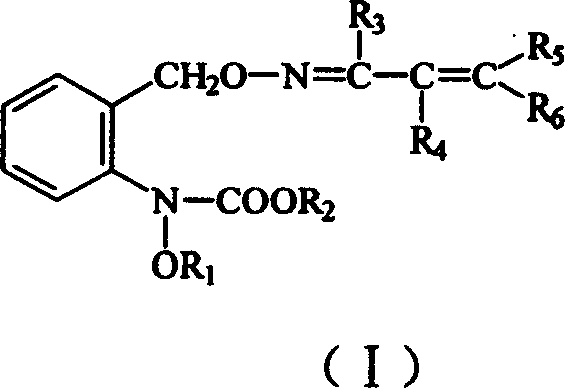
Carbamate sterilization compound containing vinyl oxime ether
A technology of carbamates and vinyl oximes, which is applied in the direction of fungicides, biocides, and chemicals for biological control, etc., and can solve the problems that there are no literature reports on carbamate fungicidal active compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of the compound N-methoxy-N-2-[1-(2-phenylvinyl) ethyleneiminooxymethyl] phenyl carbamate (compound 1)
[0022] In 20ml of toluene, add 0.64g (4mmol) of 1-(2-phenylvinyl)-acetaldoxime and 0.25g (5mmol) of 60% NaH, add 1.37g( 5.0 mmol) of N-(2-bromomethylphenyl)-N-methoxy-carbamic acid methyl ester in toluene. After dripping, react at this temperature for 10-15 minutes, and wash the reactant with water until it is neutral and anhydrous Na 2 SO 4 Dry, desolvate under reduced pressure to obtain a crude product. The crude product was washed by column chromatography with a mixture of ethyl acetate and petroleum ether (1:3) to obtain 0.54 g of the target compound with a yield of 38.3%.
[0023] Target compound 1 H NMR(300MHZ, CDCl 3 ): 2.14 (s, 3H, CH3), 3.77, 3.79 (2s, 6H, 2OCH3), 5.25 (s, 2H, CH2O), 6.78 ~ 6.91 (q, 2H, CH = CH), 7.25 ~ 7.55 (m, 9H, Ar-H)
Embodiment 2
[0025] Preparation of N-methoxy-N-2-[1-(2-(3-chlorophenyl)-vinyl) ethyleneiminooxymethyl] phenyl carbamate (compound 3)
[0026] In 20ml of dimethylformamide, add 0.92g (5mmol) of 1-(2-(3-chlorophenyl)vinyl)-acetaldoxime, 0.42g (3mmol) potassium carbonate, 1.37g (5mmol) N -(2-Bromomethylphenyl)-N-methoxy-carbamic acid methyl ester. Stir at 25-30°C for 1 hour. After the reaction is complete, the reaction solution is poured into 100ml of water and extracted twice with ethyl acetate. The ethyl acetate layer is washed with water until it is neutral, and then with anhydrous Na 2 SO 4 After drying and removing the solvent, a crude product is obtained. The crude product was washed by column chromatography with a mixture of ethyl acetate and petroleum ether (1:3) to obtain 1.16 g of the target compound with a yield of 60%.
[0027] Target compound 1 H NMR(300MHZ, CDCl 3 ): 2.12(s, 3H, CH3), 3.76, 3.79(2s, 6H, 2×OCH3), 5.25(s, 2H, CH2O), 6.79(s, 2H, CH=CH), 7.24~7.53(m, 8H, Ar-H)
Embodiment 3
[0029] Preparation of N-methoxy-N-2-[1-(2-(4-chlorophenyl)-vinyl) ethyleneiminooxymethyl] phenyl carbamate (compound 4)
[0030] In 20ml dimethylformamide, add 0.92g (5mmol) of 1-(2-(4-chlorophenyl)vinyl)-acetaldoxime, 0.42g (3mmol) potassium carbonate, 1.37g (5mmol) N-(2-Bromomethylphenyl)-N-methoxy-carbamic acid methyl ester. Stir at 25~30℃ for half an hour. After the reaction is complete, the reaction solution is poured into 100ml of water and extracted twice with ethyl acetate. The ethyl acetate layer is washed with water until it is neutral, and then with anhydrous Na 2 SO 4 After drying and removing the solvent, a crude product is obtained. The crude product was washed by column chromatography with a mixture of ethyl acetate and petroleum ether (1:3) to obtain 1.22 g of the target compound with a yield of 62%.
[0031] Target compound 1 H NMR(300MHZ, CDCl 3 ): 2.09(s, 3H, CH3), 3.75, 3.76(2s, 6H, 2×OCH3), 5.21(s, 2H, CH2O), 6.82, 6.88(d, 2H, CH=CH), 7.27~7.55( m, 8H, Ar-H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com