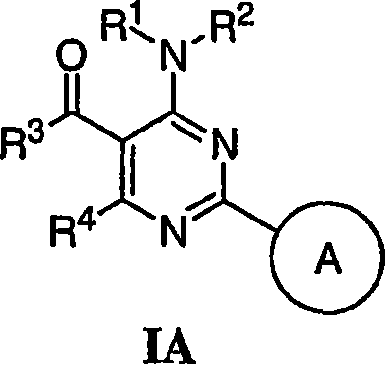
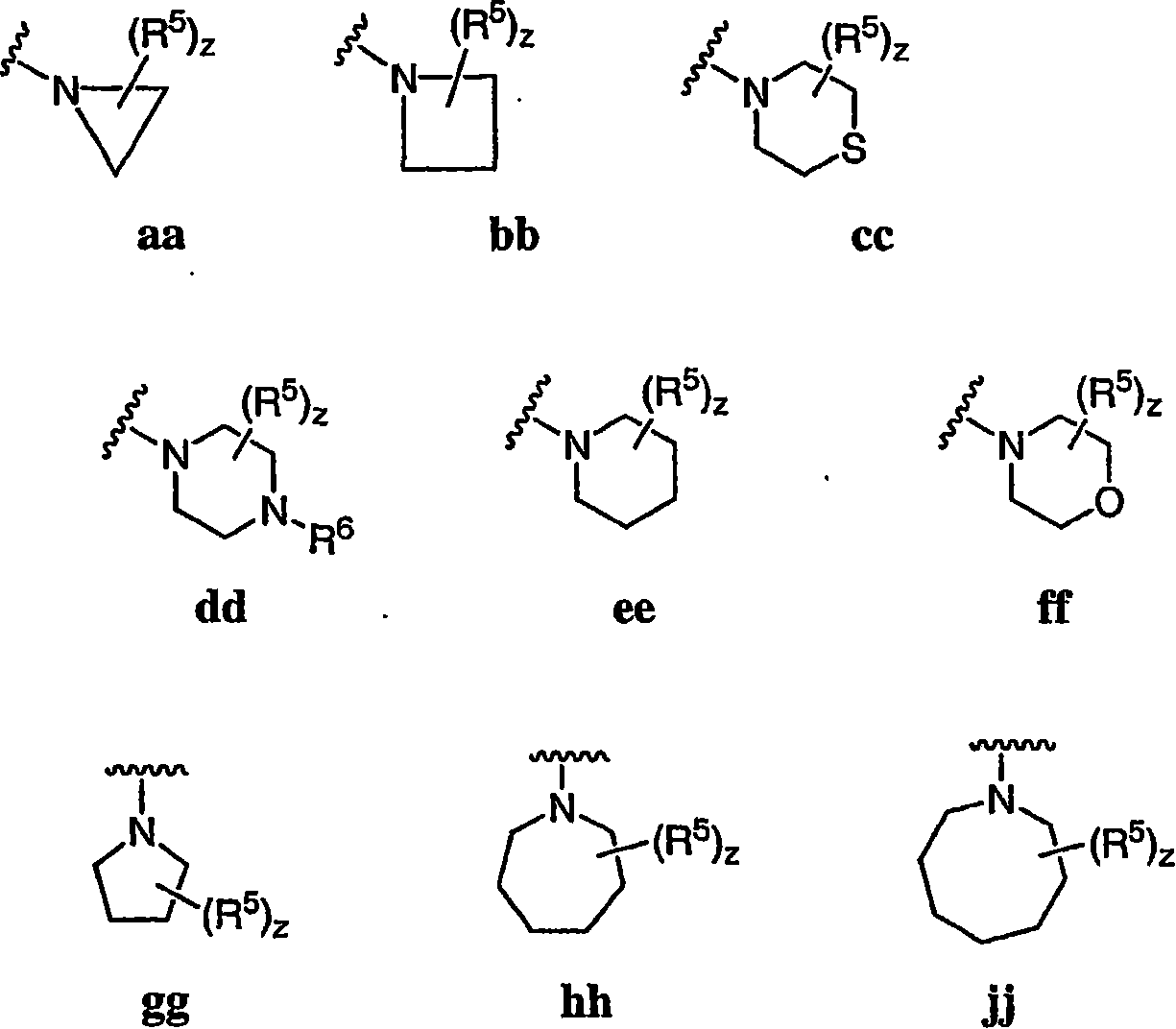
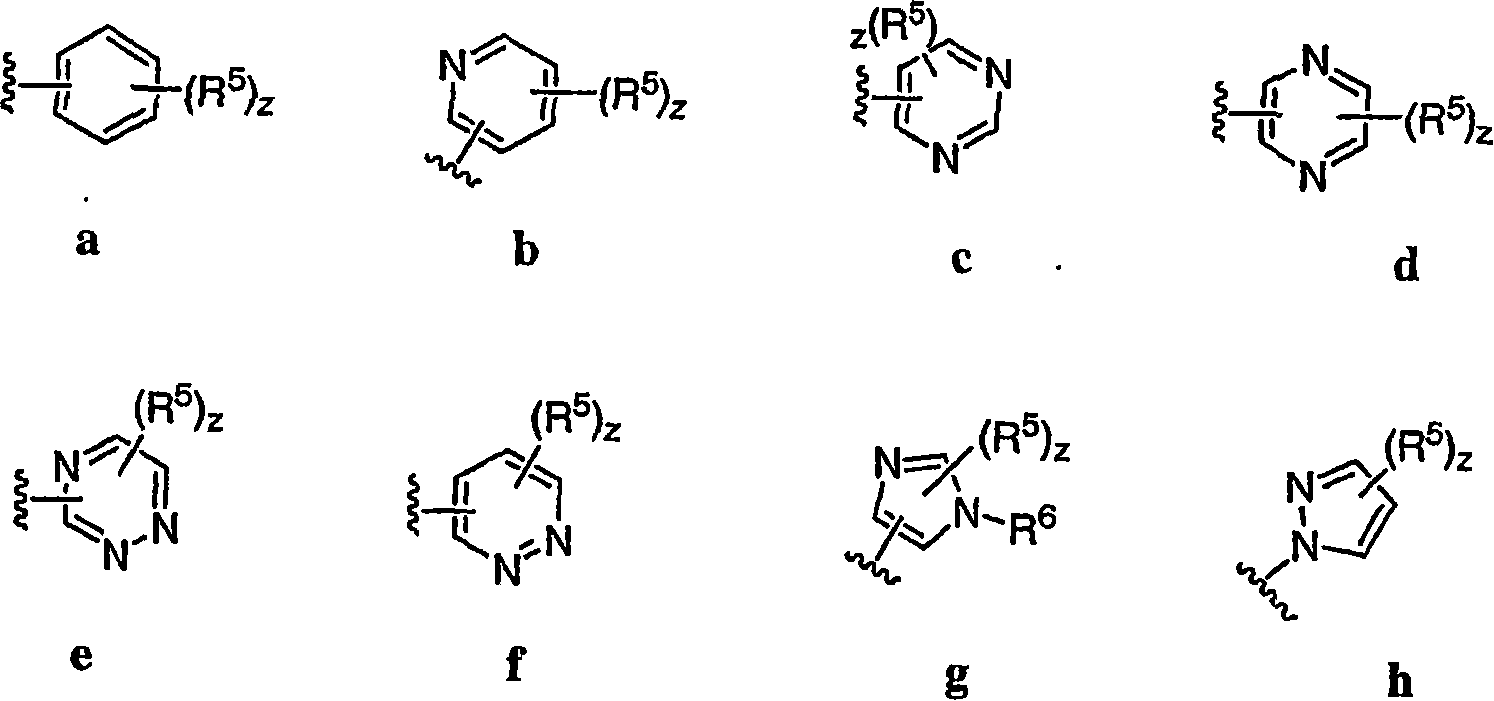
Pyrimidines useful as modulators of voltage-gated ion channels
A heteroatom, nitrogen atom technology, applied in the field of pyrimidines that can be used as voltage-gated ion channel regulators, can solve problems such as side effect limitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0519]
[0520] In a 250 mL three-necked round bottom flask equipped with a magnetic stirrer and reflux condenser, N , N-Dimethylaniline (2.10 mL, 16.3 mmol). The suspension was heated at reflux for 30 minutes, slowly forming a clear solution. The solution was concentrated under reduced pressure and the residue was poured on ice (100 g). The solution was basified to pH = 9.0 with concentrated aqueous sodium bicarbonate. Make the mixture in CH 2 Cl 2 with H 2 O distribution. The organic solution was dried (MgSO 4 ), evaporated to dryness under reduced pressure. The residue was dissolved in anhydrous THF (30 mL), cooled to 0°C. Dimethylaniline (16.3 mL, 32.6 mmol, 2.0 M in THF) was added dropwise over 10 minutes with stirring. The solution was then stirred at 0 °C for 1 hour. The solution was concentrated under reduced pressure and the residue was purified by silica gel chromatography eluting with 90% hexane / 10% ethyl acetate to afford 2 (1.70 g, 7.34 mmol, 45% yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com