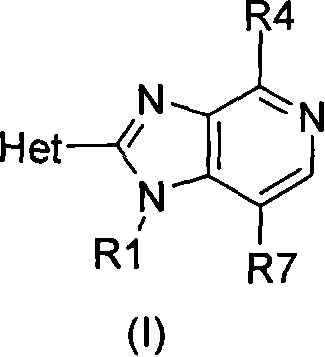
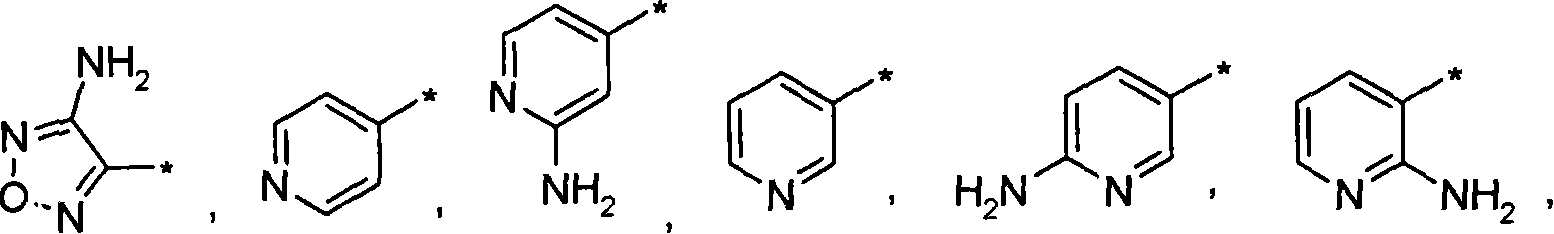
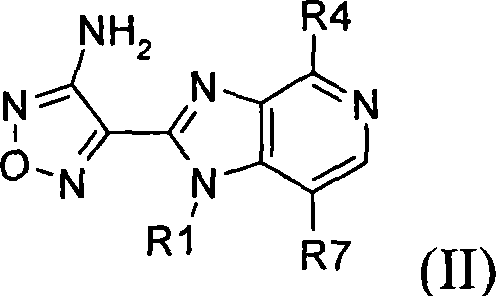
Inhibitors of Akt activity
A technology selected from, cycloalkyl, applied in the direction of organic active ingredients, medical preparations containing active ingredients, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0474] In the preparation of the compound of formula (II) of the present invention, the following novel intermediates were prepared.
[0475] 4-(7-bromo-4-chloro-1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-oxadiazol-3-amine, which is Intermediate that can be prepared in Example 18(e).
[0476] 2-(4-amino-1,2,5-oxadiazol-3-yl)-1-methyl-4-phenyl-1H-imidazo[4,5-c]pyridine-7-carboxylic acid, It is an intermediate that can be prepared in Example 98(g).
[0477] In the preparation of the compounds of formula (I) of the present invention, the following novel intermediates were prepared.
[0478] Ethyl 4-chloro-5-nitro-6-phenyl-3-pyridinecarboxylate, an intermediate that could be prepared in Example 98(d).
[0479] The present invention also relates to a method for preparing a compound of formula (I) and / or pharmaceutically acceptable salts, hydrates, solvates and prodrugs thereof, the method comprising 4-chloro-5-nitro-6-phenyl- Ethyl 3-pyridinecarboxylate is converted to a comp...
Embodiment 1
[0574] Preparation of 4-(4-phenyl-1-piperidin-4-yl-1H-imidazo[4,5-c]pyridin-2-yl)-furazan-3-ylamine trifluoroacetate
[0575] a) (1-benzyl-piperidin-4-yl)-(3-nitro-pyridin-4-yl)-amine
[0576] 4-Methoxy-3-nitropyridine (4.34g, 28.1mmol), 4-amino-1-benzylpiperidine (6.01g, 30.9mmol), and NaOAc (2.31g, 28.1mmol) were dissolved in anhydrous The mixture in ethanol (20 mL) was stirred at reflux for 54 hours. The reaction mixture was cooled to ambient temperature and concentrated in vacuo. Dissolve the residue in CH 2 Cl 2 (100 mL) and washed with water (2×30 mL). The organic layer was passed through anhydrous MgSO 4 Drying and concentration in vacuo gave the product (8.78g) as a dark yellow solid. 1 H NMR (400MHz, CDCl 3 )δ9.21 (s, 1H), 8.26 (dd, J=6.0, 0.4Hz, 1H), 8.20 (wide d, J=7.1Hz, 1H), 7.34-7.25 (complex m, 5H), 6.70 ( d, J=6.0Hz, 1H), 3.62-3.53(m, 1H), 3.55(s, 2H), 2.89-2.79(m, 2H), 2.30-2.20(m, 2H), 2.10-2.00(m, 2H), 1.76-1.65 (m, 2H).
[0577] b)N 4 -(1-Benzyl-...
Embodiment 2
[0588] 4-[4-(3-Chloro-phenyl)-1-piperidin-4-yl-1H-imidazo[4,5-c]pyridin-2-yl]-furazan-3-ylamine hydrochloride salt preparation
[0589] By substituting 3-chlorophenylboronic acid for the phenylboronic acid in Example 1(d) and the method described in Examples 1(e) to 1(f), and trituration with 4N HCl / dioxane , to give the title compound. MS(ES+)m / z 396.0[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com