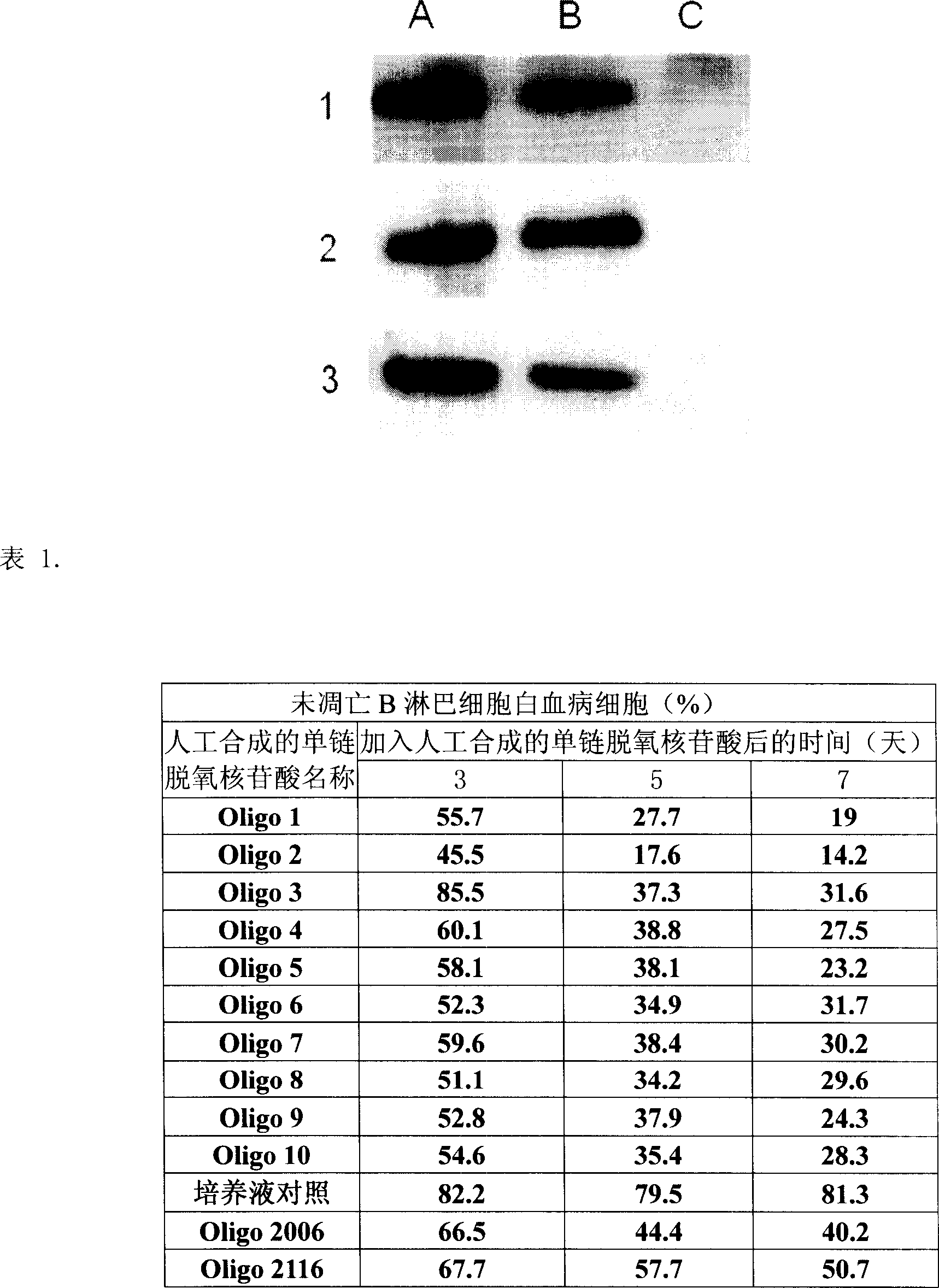
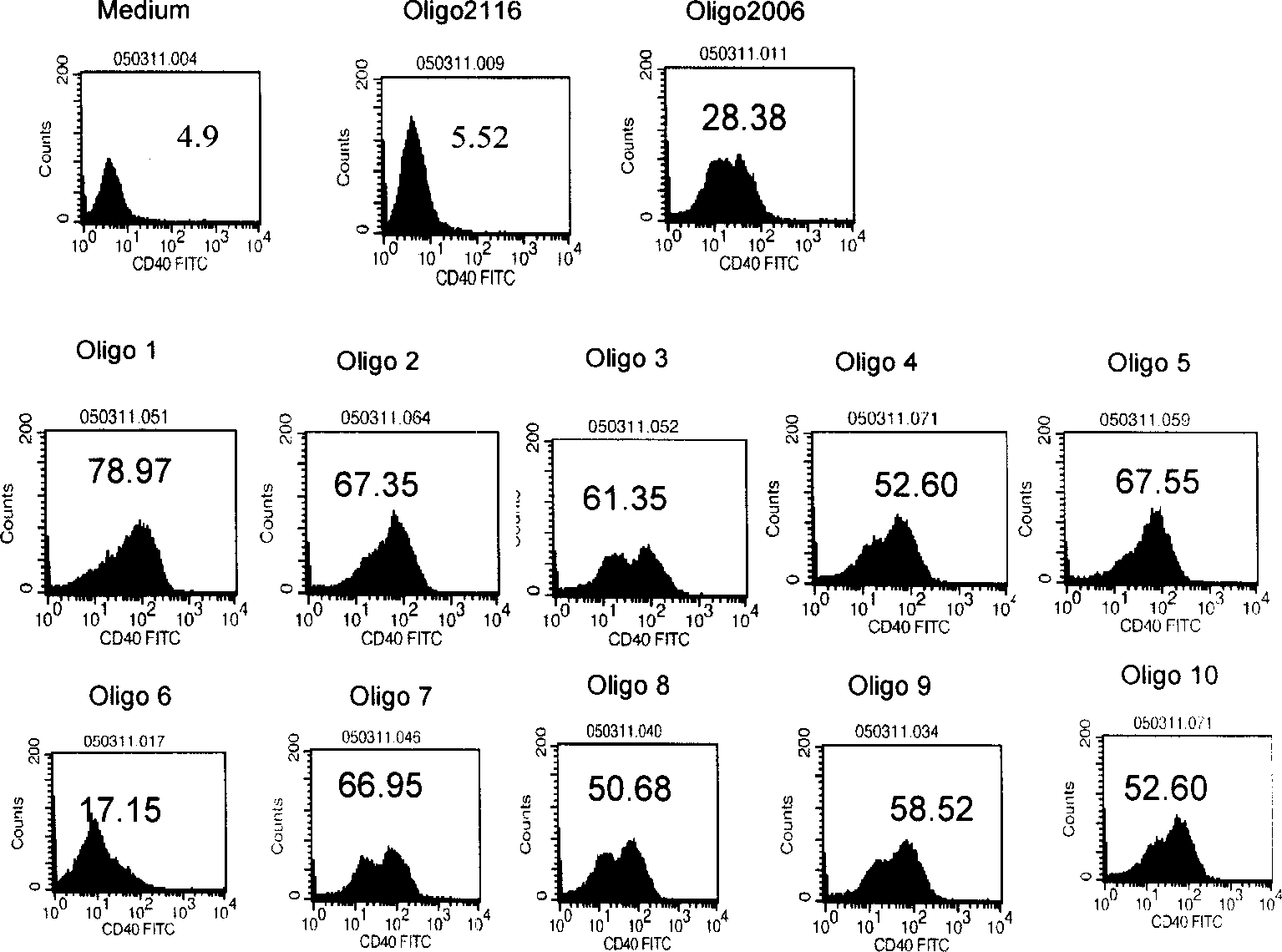
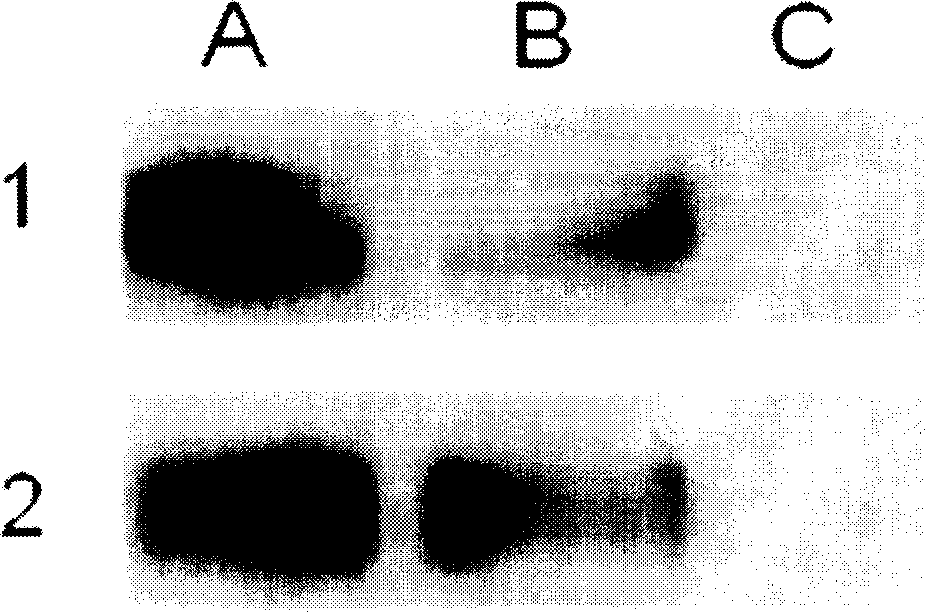
Artificial single-chain deoxynucleotide having therapeutic effect to human B cell tumour
A deoxynucleotide and artificial synthesis technology, which is applied in the field of preparations for the treatment of human B lymphocyte tumors, can solve the problems of growth inhibition of B lymphocyte tumor cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the design of the artificially synthesized single-stranded deoxynucleotide
[0044] The design sequence is as follows:
[0045] 1. The artificially synthesized single-stranded deoxynucleotide (X1=A, T, G; X2=A, T; Y1=A, T; Y2=A, T, C; L, M=A, T, C, G; n is 0-6)
[0046] 5'-ggggTCgTTCgTCgTTgggggg-3' (SEQ ID NO: 1) [121]
[0047] 5'-ggggATAACgTTgCgggggg-3' (SEQ ID NO: 2) [143]
[0048] 5'-ggggTgCAACgTTCAgggggg-3' (SEQ ID NO: 3) [402]
[0049] 5'-ggggTCCTACgTAggAgggggg-3' (SEQ ID NO: 4) [123]
[0050] 5'-ggggTCCATgACgTTCCTgAAgggggg-3' (SEQ ID NO: 5) [603]
[0051] 5'-gggggACgTCgCCgggggggg-3' (SEQ ID NO: 6) [118]
[0052] 5'-ggATCCgTACgCATgggggg-3' (SEQ ID NO: 7) [320]
[0053] 5'-ggggggAATCgATTCgggggg-3' (SEQ ID NO: 8) [154]
[0054] 5'-gggATgCATCgATgCATCgggggg-3' (SEQ ID NO: 9) [464]
[0055] 5'-ggTgCgACgTCgCAgggggg-3' (SEQ ID NO: 10) [471]
[0056] 5'-gggACgTACgTCgggggg-3' (SEQ ID NO: 11) [390]
[0057] 5'-gggggATCgACgTCgATCgggggg-3' (SEQ ID NO: ...
Embodiment 2
[0235] Embodiment 2, the synthesis of single-stranded deoxynucleotide
[0236] The solid-phase phosphoramidite triester method is used to synthesize DNA fragments. This method has the advantages of high efficiency and rapidity, and has been widely used in DNA chemical synthesis.
[0237] DNA chemical synthesis is different from enzymatic DNA synthesis. The enzymatic DNA synthesis process extends from the 5'→3' direction, while DNA chemical synthesis starts from the 3' end. Concrete reaction steps are as follows:
[0238] 1. Deprotection group
[0239] Use trichloroacetic acid to remove the protective group DMT (dimethoxytrityl) of the nucleotide attached to CPG (Controlled Pore Glass) to obtain a free 5'-hydroxyl terminal for the next condensation reaction use.
[0240] 2. Activation
[0241] The phosphoramidite-protected nucleotide monomer is mixed with tetrazolium activator and enters the synthesis column to form the phosphoramidite tetrazolium active intermediate (its 3...
Embodiment 3
[0249] Example 3, Expression of Toll-like receptor 9 in human B lymphocytic leukemia cells
[0250] 1. Preparation of human chronic B lymphocytic leukemia cells
[0251] Peripheral blood mononuclear cells (PBMC) were obtained from the blood of human chronic B lymphocytic leukemia patients by Ficoll-hypaque gradient centrifugation. CD5 was isolated using B lymphocyte isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) + CD19 + CD23 + Human chronic B lymphocytic leukemia cells with a purity of 95%. The specific operation was carried out according to the method provided by Miltenyi Biotec Company. Using the B or T lymphocyte isolation kit (MiltenyiBiotec, Bergisch Gladbach, Germany), the B or T lymphocytes isolated from normal human PBMC, the obtained B lymphocytes (CD19 + ) and the obtained T lymphocytes (CD3 + ) have a purity of more than 95%. The specific operation was carried out according to the method provided by Miltenyi Biotec (Bergisch Gladbach, Germany). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com