Proteases
A technology of protease and protease activity, applied in protein food processing, protein food ingredients, hydrolytic enzymes, etc., can solve the problem of no sequence information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0236] In alternative embodiments, the term "alter" is used instead of "substitute" as a general term for alteration in protein molecules. This alternative embodiment includes each claim to illustrate claim 1, and specifically includes anything described herein, such as definitions (except definitions substituted), ie aspects, particular embodiments, etc.
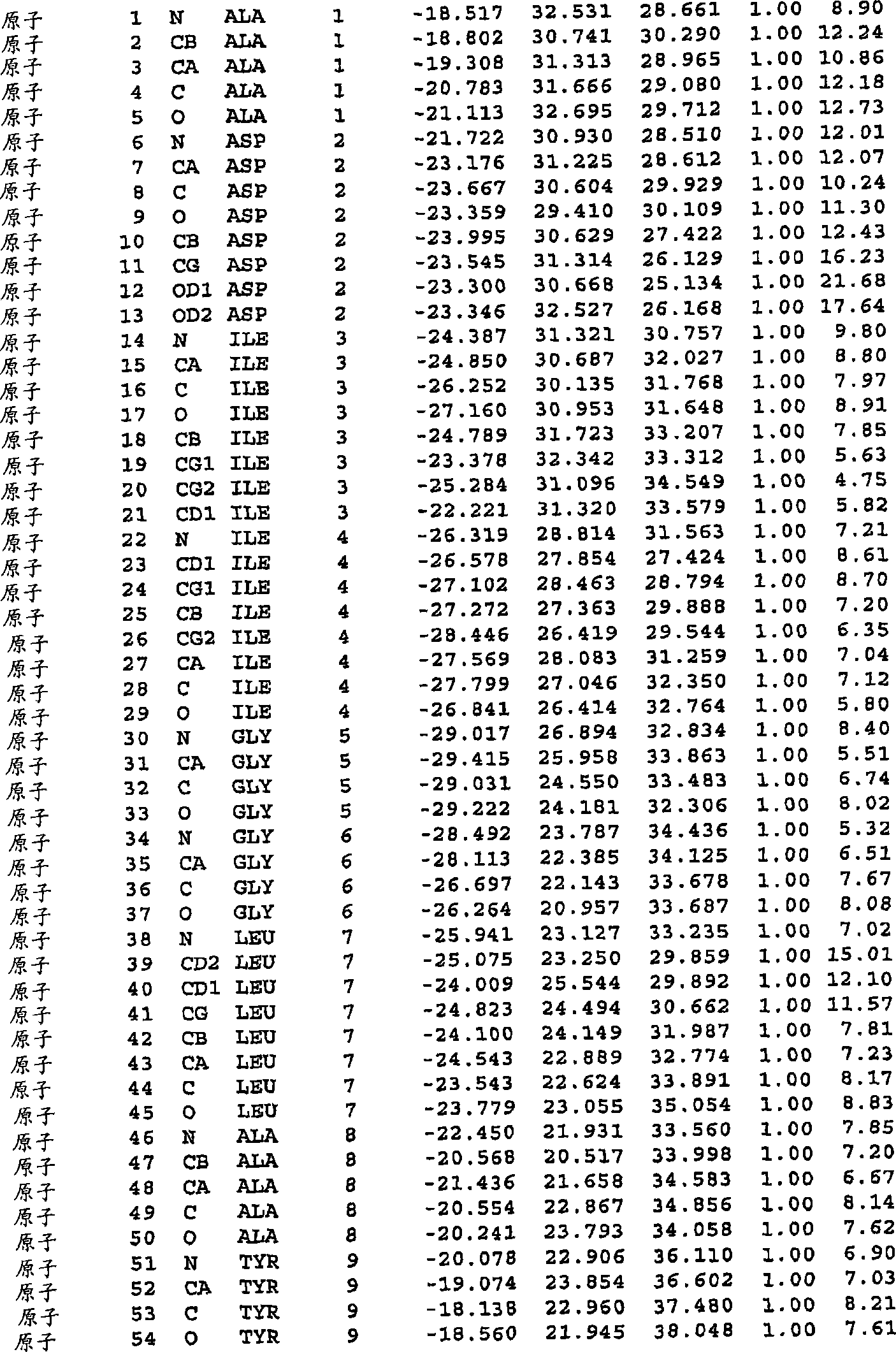
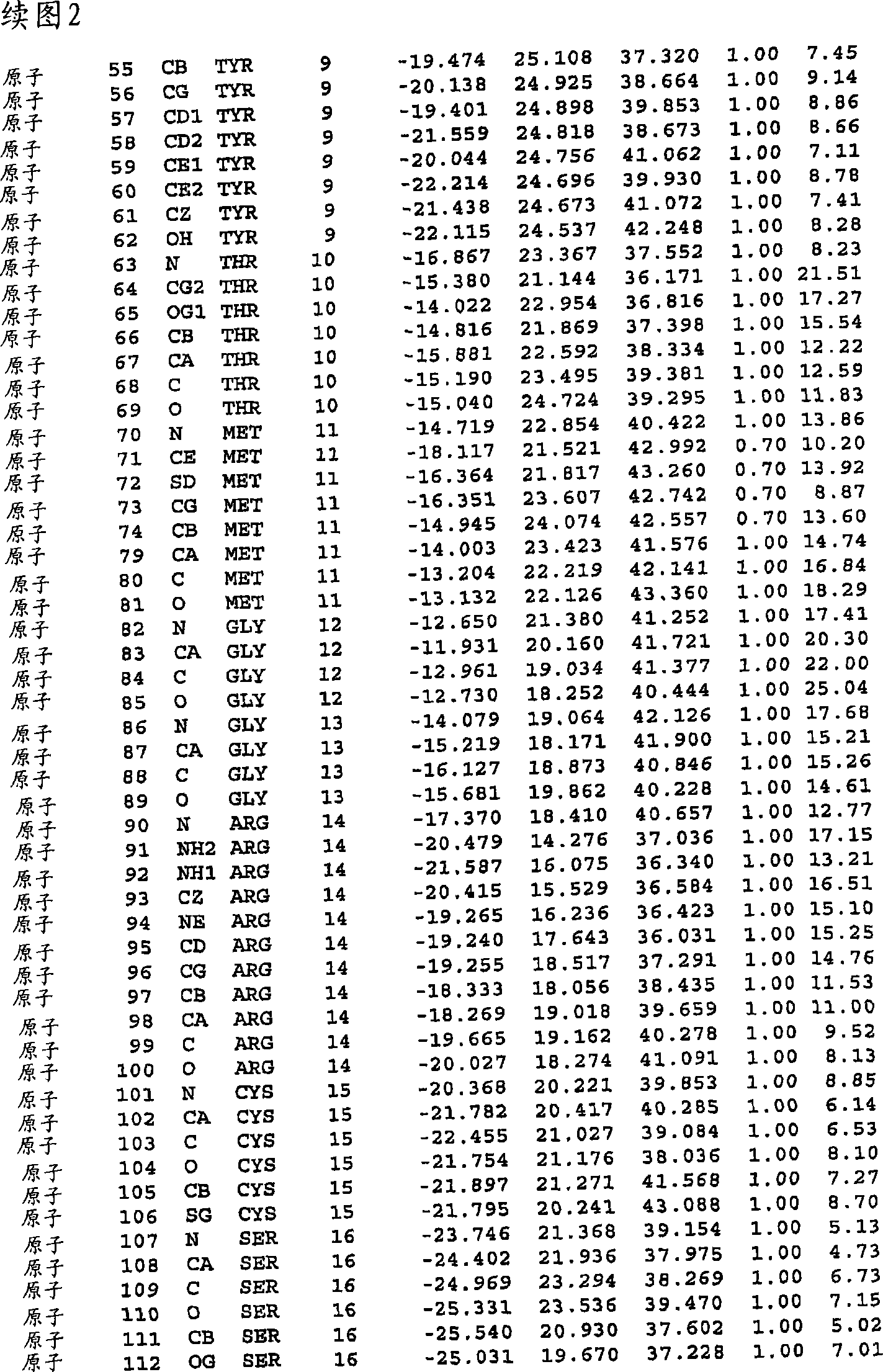
[0237] A variant of the parent protease comprising an alteration in at least one position of at least one region selected from the group consisting of:
[0238] 6-18; 22-28; 32-39; 42-58; 62-63; 66-76; 78-100; 103-106; 111-114; 151; 155-156; 160-176; 179-181; and 184-188; of which
[0239] (a) said change is independently
[0240] (i) an amino acid insertion immediately downstream of said position,
[0241] (ii) a deletion of the amino acid occupying said position, and / or
[0242] (iii) substitution of the amino acid occupying said position;
[0243] (b) the variant has protease activity; and
[0244] (c) each positio...
Embodiment 1
[0300] Example 1: Protease Assay
[0301] pNA assay
[0302] pNA substrate: Suc-AAPF-pNA (Bachem L-1400).
[0303] Temperature: room temperature (25°C)
[0304] Assay buffer: 100mM succinic acid, 100mM HEPES, 100mM CHES, 100mM CABS, 1mM CaCl 2 , 150 mM KCl, 0.01% Triton X-100, which was adjusted to pH 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, and 12.0 with HCl or NaOH.
[0305] 20 μl protease (diluted in 0.01% Triton X-100) was mixed with 100 μl assay buffer. The assay was started by adding 100 [mu]l pNA substrate (50 mg dissolved in 1.0 ml DMSO and further diluted 45x with 0.01% TritonX-100). The increase in OD405 was monitored as a measure of protease activity.
[0306] Protazyme AK Assay
[0307] Substrate: Protazyme AK sheet (cross-linked and stained casein; from Megazyme)
[0308] Temperature: controlled (analysis temperature).
[0309] Assay buffer: 100mM succinic acid, 100mM HEPES, 100mM CHES, 100mM CABS, 1mM CaCl 2 , 150 mM KCl, 0.01% Trito...
Embodiment 2
[0311] Example 2: Preparation and detection of protease variants
[0312] Four protease variants comprising the amino acid sequence of amino acids 1-188 of SEQ ID NO: 2 (Protease 10), wherein said amino acid sequences contain the single substitutions N47D, T127R, N92K, and Q54R, respectively, as described below for variant N47D Prepared as described.
[0313] Site-directed mutagenesis was performed using the Mega-primer method as described by Sarkar and Sommer, 1990 (BioTechniques 8:404-407).
[0314] The N47D variant was constructed by using the following primers, where primer R10WT-CL29 (SEQ ID NO: 11 ) is gene specific and primer RSWT126 (SEQ ID NO: 12) is mutagenic:
[0315] R10WT-CL29: 5'CCGATTATGGAGCGGATTGAACATGCG 3' (SEQ ID NO: 11)
[0316] RSWT126: 5'GTGACCATCGGCGACGGCAGGGGCGTCTTCG 3' (SEQ ID NO: 12),
[0317] A DNA fragment of approximately 469 bp was amplified by PCR from the construct described below.
[0318] The Protease 10 DNA construct used for the amplifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com