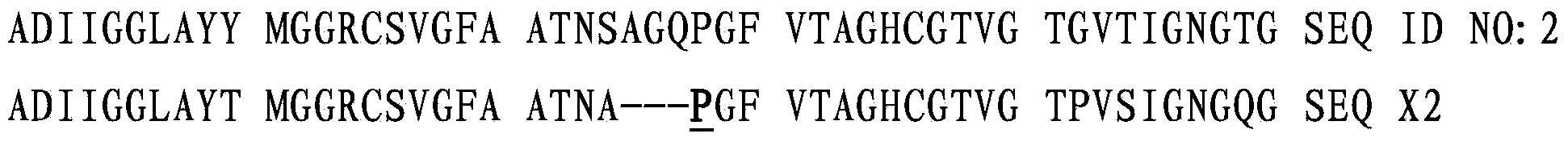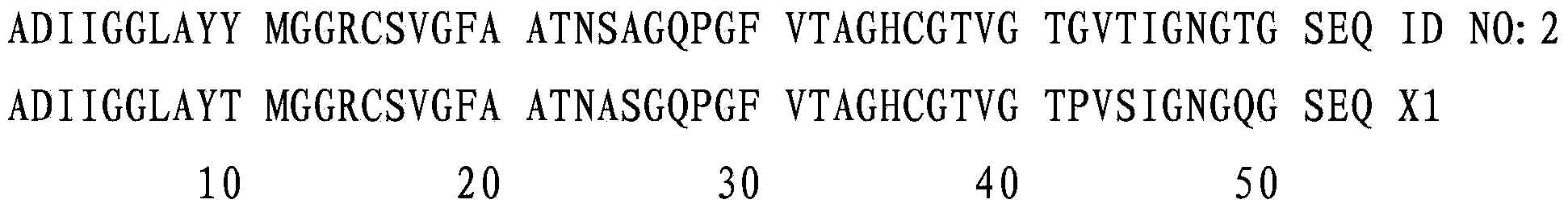Proteases
A protease activity, amino acid technology, applied in the direction of enzymes, hydrolases, enzymes, etc., can solve the problem of no sequence information, strains can not be obtained and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0421] Example 1: Cloning and expression of the protease from Nocardiopsis darsonville subsp. darsonville subsp. DSM43235
[0422] Reagents and media
[0423]
[0424] 60g thiocyanate, 20ml 0.5M EDTA, pH8.0, 20ml H 2 O dissolves at 65°C. Cool to room temperature (RT) and add 0.6 g N-lauryl sarcosine. Add H 2 0 to 100ml and filter through a 0.2μ sterile filter.
[0425] NH 4 Ac 7.5M CH 3 COONH 4
[0426] TER 1 μg / ml RNase A in TE-buffer
[0427] CIA Chloroform / Isoamyl Alcohol 24:1
[0428] Experimental procedure
[0429] SEQ ID NO: 1 is the DNA sequence encoding the proform of the protease from Nocardiopsis darsonville subsp. darsonville subsp. DSM 43235. Nucleotides 499-1062 correspond to the mature peptide coding portion.
[0430] SEQ ID NO:2 is the deduced amino acid sequence of SEQ ID NO:1. Amino acids -166 to 1 are the propeptide, while amino acids 1 to 188 are the mature peptide.
[0431] Clone SEQ ID NO:1
[0432] The wild type was grown at 30°C f...
Embodiment 2
[0452] Example 2: Purification and Characterization of Protease from Nocardiopsis darsonville subspecies DSM43235
[0453] protease assay
[0454] 1) pNA determination:
[0455] pNA substrate: Suc-AAPF-pNA (Bachem L-1400)
[0456] Temperature: Room temperature (25°C)
[0457] Assay buffer: 100mM succinic acid, 100mM HEPES, 100mM CHES, 100mMCABS, 1mM CaCl 2 , 150mm KCl, 0.01% Triton X-100, adjusted to pH 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0 and 12.0 with HCl or NaOH.
[0458] 20 μl protease (diluted in 0.01% Triton X-100) was mixed with 100 μl assay buffer. The assay was started by adding 100 μl of pNA substrate (50 mg dissolved in 1.0 ml DMSO and further diluted 45× with 0.01% TritonX-100). Monitor OD 405 The increase in is used as a measure of protease activity.
[0459] 2) Protazyme AK determination:
[0460] Substrate: Protazyme AK sheet (cross-linked and stained casein; from Megazyme)
[0461] Temperature : Controlled (measurement tempera...
Embodiment 3
[0481] Example 3: Performance of Nocardiopsis darsonville subsp. darsonville subsp. DSM43235 protease in a monogastric in vitro digestion model
[0482] Purified preparations of the mature fraction of the protease having SEQ ID NO: 2 (prepared as described in Examples 1 and 2) were tested in an in vitro model simulating digestion in monogastric animals. Specifically, the ability of the proteases to improve solubilization and digestion of corn / -SBM (corn / -soybean meal) proteins was tested. In the table below, this protease is referred to as "protease of the invention". The in vitro system consisted of 15 shake flasks in which the maize / -SBM substrate was initially incubated with HCl / pepsin - simulating gastric digestion - and subsequently incubated with trypsin - simulating Intestinal digestion. Ten of the shake flasks were formulated with protease at the beginning of the stomach phase, while the remaining shake flasks served as blanks. At the end of the incubation period of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com