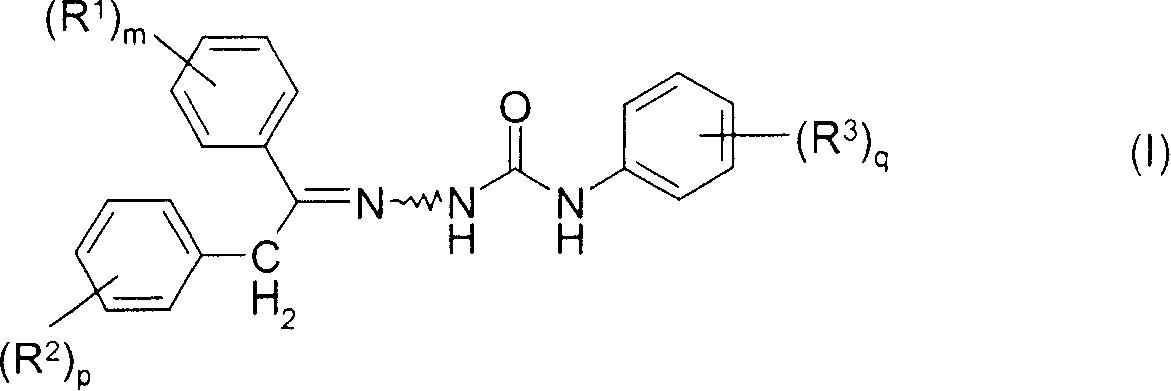
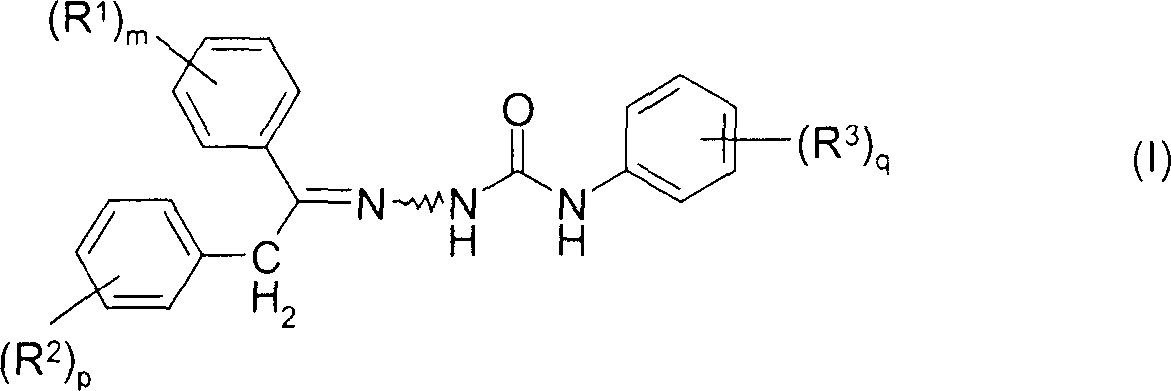
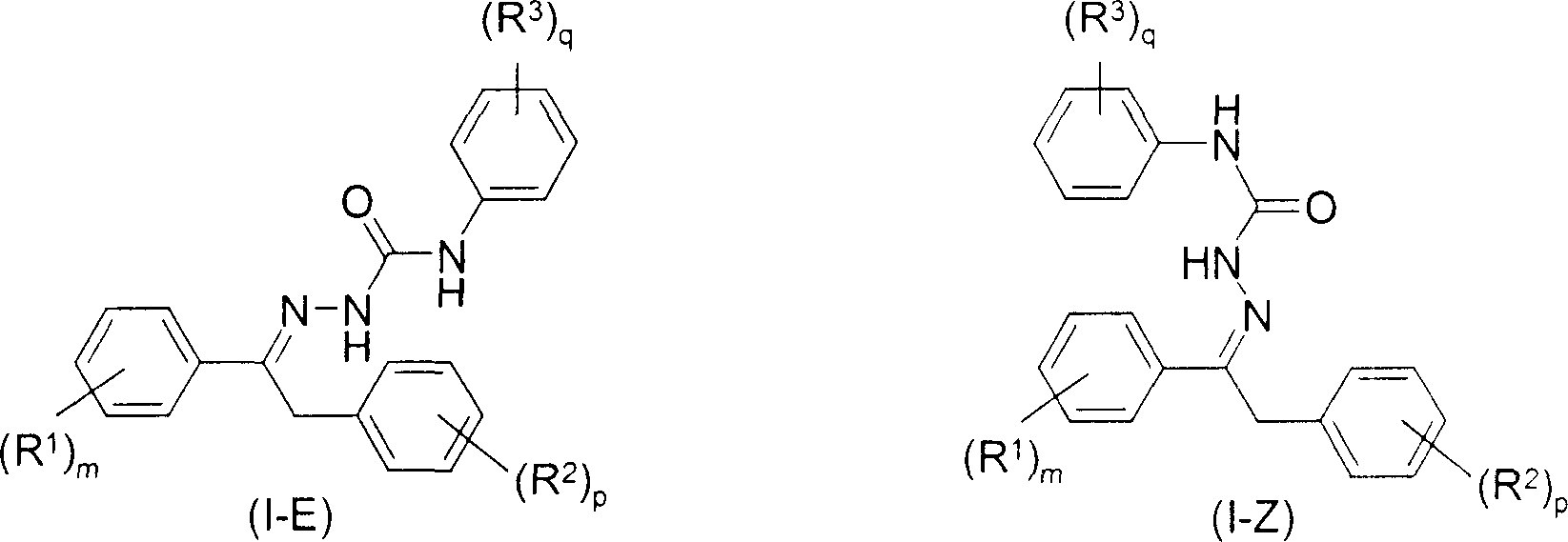
Cis-trans isomerisation of semicarbazone compounds
一种化合物、异构化的技术,应用在旋光化合物分离、有机化学、有机化学方法等方向,能够解决不具有E/Z比、低产率分离产物、结晶费时等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Conversion of the pure Z-form of compound I.1 into its E-form
[0050] A mixture of 2 g of the Z-isomer I.1-Z and 0.04 g of iodine was heated at 90° C. in a sealed tube. Liquid chromatography (see below) indicated that the product contained 97.8% of the E-isomer I.1-E and 2.2% of the Z-isomer I.1-Z (E / Z ratio 97.8:2.2). Iodine was then removed under vacuum at 90°C for 2 hours. The recovery yield was 100%. No other impurities were detected by liquid chromatography.
[0051] Liquid chromatography: column: reverse phase RP 8-column, Kromasil 100-3.5C8; unit: acetonitrile / (water+0.1% trifluoroacetic acid, pH 2.4) gradient; detection: UV 2235.4nm.
Embodiment 2
[0053] Treatment of crude reaction mixture containing 97.3% of compound I.1 with an E / Z ratio of about 4.9:1
[0054] 2 g of a solid containing about 97.3% of compound I.1 with an E / Z ratio of about 4.9:1 and 0.04 g of iodine were heated in a sealed tube at 90° C. for 2 hours. Iodine was then removed in vacuo by drying overnight at 90°C. The recovery yield was about 100%. No other impurities were detected. The product contained 95.9% by weight of the E-isomer I.1-E and 1.4% by weight of the Z-isomer I.1-Z, determined by liquid chromatography (E / Z ratio 68.5:1).
Embodiment 3
[0056] Conversion of pure Z-form compound I.1
[0057] 2 g of compound I.1-Z and 0.1 g of iodine were suspended in 8 g of chlorobenzene and the resulting slurry was heated at 60° C. for 6 hours. The reaction mixture was then cooled and 10 g of hexane was added. The reaction product was filtered and dried overnight in an oven at 70 °C. 1.8 g of product are thus obtained. The resulting E / Z ratio is about 12:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| process yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com