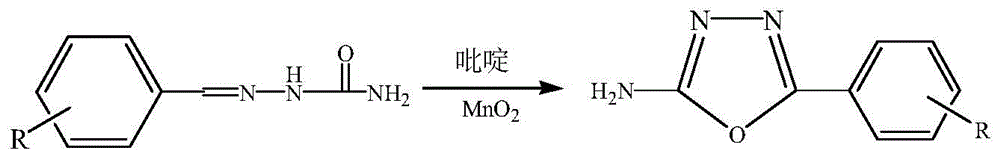
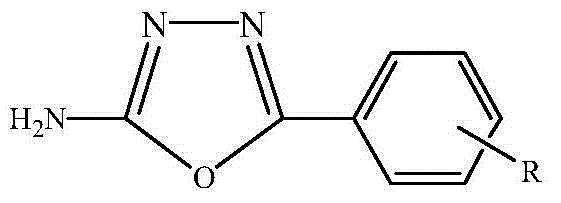
2-amido-5-substituted-1,3,4-oxadiazole as well as preparation method and application thereof
A technology of oxadiazole and amino, which is applied in the field of chemical synthesis, can solve problems such as side reactions, increased costs, and incomplete reactions, and achieve the effects of promoting reactions, simplifying post-treatment operations, and good antibacterial effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Add Amol of 2-benzylidene semicarbazide, Bmol of manganese dioxide and CmL of pyridine to a dry three-necked flask, stir and react at 110°C, monitor the reaction with TLC during the reaction until the raw material point of semicarbazone Disappear, obtain reaction mixture; Wherein, A:B:C=1:1.2:10; The developer used during TLC monitoring is to be mixed with ethyl acetate and sherwood oil, and the volume ratio of ethyl acetate and sherwood oil 1:3;
[0034] 2) The reaction mixture was cooled to room temperature, filtered under reduced pressure, and the obtained filtrate was concentrated to dryness to obtain a white solid, which was washed with water and filtered under reduced pressure to obtain a crude product, which was recrystallized with absolute ethanol to obtain 2 -Amino-5-phenyl-1,3,4-oxadiazole pure product, the yield can reach more than 93%.
[0035] m.p.=153~154℃; IR(KBr, ν / cm -1 ): 3396.24, 3301.59, 1655.72, 1325.30, 1124.73.
Embodiment 2
[0037] 1) Add Amol of 2-(2'-chlorophenyl)methylene semicarbazide, Bmol of manganese dioxide and CmL of pyridine to a dry three-necked flask, stir and react at 110°C, monitor the reaction with TLC during the reaction Until the raw material point of semicarbazone disappears, a reaction mixture is obtained; wherein, A:B:C=1:1.2:10; the developing agent used during TLC monitoring is formed by mixing ethyl acetate and sherwood oil, and ethyl acetate The volume ratio of ester and petroleum ether is 1:3;
[0038]2) The reaction mixture was cooled to room temperature, filtered under reduced pressure, and the obtained filtrate was concentrated to dryness to obtain a white solid, which was washed with water and filtered under reduced pressure to obtain a crude product, which was recrystallized with absolute ethanol to obtain 2 -Amino-5-(2'-chlorophenyl)-1,3,4-oxadiazole pure product, the yield can reach more than 93%.
[0039] The determination data of 2-amino-5-(2'-chlorophenyl)-1,3,4...
Embodiment 3
[0043] 1) Add Amol of 2-(2'-hydroxyphenyl)methylene semicarbazide, Bmol of manganese dioxide and CmL of pyridine to a dry three-necked flask, stir and react at 100°C, monitor the reaction with TLC during the reaction Until the raw material point of semicarbazone disappears, a reaction mixture is obtained; wherein, A:B:C=1:1:12; the developing agent used during TLC monitoring is formed by mixing ethyl acetate and sherwood oil, and ethyl acetate The volume ratio of ester and petroleum ether is 1:3;
[0044] 2) The reaction mixture was cooled to room temperature, filtered under reduced pressure, and the obtained filtrate was concentrated to dryness to obtain a white solid, which was washed with water and filtered under reduced pressure to obtain a crude product, which was recrystallized with absolute ethanol to obtain 2 -Amino-5-(2'-hydroxyphenyl)-1,3,4-oxadiazole pure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com