Jinganmin oral disintegrant tablets and preparation thereof
A technology for Jin-sensitivity oral and orally disintegrating tablets, which is applied in the field of Jin-sensitivity orally-disintegrating tablets and their preparation, and can solve the problems of no reports of Jin-sensing orally-disintegrating tablets being found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
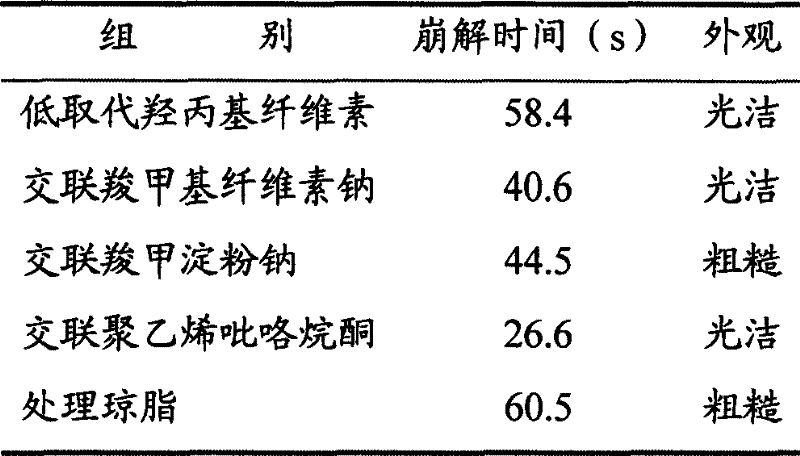
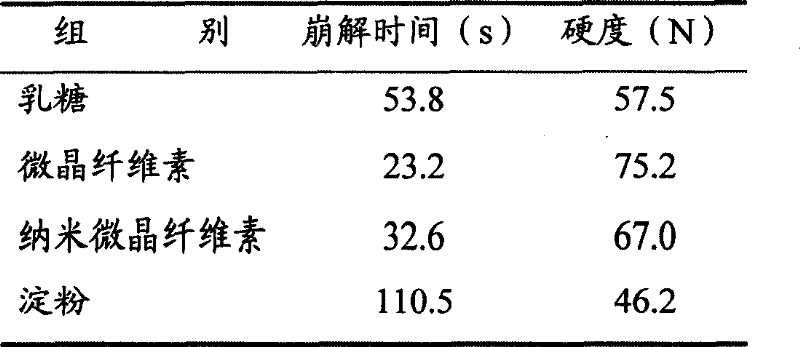
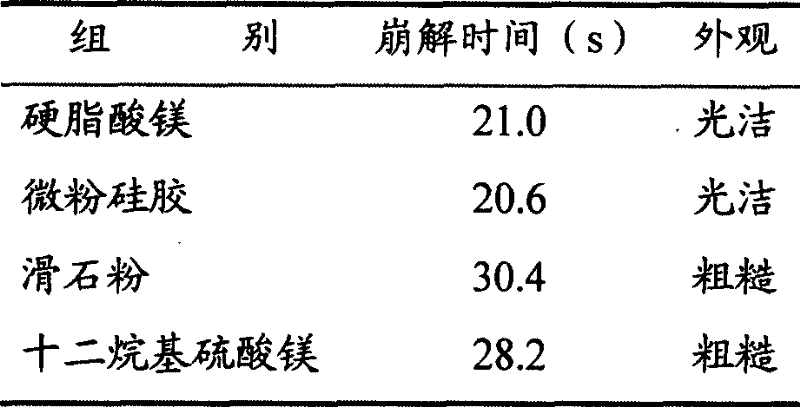
[0060] (1) Prescription: 250g of aspirin, 100g of amantadine hydrochloride, 15g of caffeine, 2g of chlorpheniramine, 10g of artificial bezoar, 500g of honeysuckle, and 500g of Daqingye.
[0061] (2) Preparation of the main ingredient: get the honeysuckle medicinal material, pulverize it, cross a 40-mesh sieve, add 70% hydrochloric acid ethanol solution of 4 times the amount of pH3 and soak for half an hour, put it into an ultrasonic extraction tank, and carry out ultrasonic vibration extraction for 20 minutes. Oscillation frequency 30kHz, extraction twice, combined extracts, filtered, filtrate under 50 ℃ under reduced pressure to recover ethanol, dried, crushed to obtain honeysuckle extract; take Daqingye medicinal material, crushed, passed through a 20 mesh sieve, added 5 times Reflux extraction with 80% ethanol for 2 times, each time for 1 hour, combine the extracts, filter, and recover the ethanol from the filtrate under reduced pressure, dry, and pulverize to obtain the Fol...
Embodiment 2
[0070] (1) Prescription: 250g of aspirin, 100g of amantadine hydrochloride, 15g of caffeine, 2g of chlorpheniramine, 10g of artificial bezoar, 500g of honeysuckle, and 500g of Daqingye.
[0071] (2) Preparation of the main ingredient: get the honeysuckle medicinal material, pulverize it, pass through a 40-mesh sieve, add 80% hydrochloric acid ethanol solution of 8 times the amount of pH4 and soak for half an hour, put it into an ultrasonic extraction tank, and carry out ultrasonic vibration extraction for 30 minutes. Oscillation frequency 40kHz, extract 3 times, combine extracts, filter, filtrate recovers ethanol under reduced pressure below 50°C, dry, grind to obtain honeysuckle extract; Reflux extraction with 90% ethanol for 2 times, 1 hour each time, combine the extracts, filter, recover the ethanol from the filtrate under reduced pressure, dry, and pulverize to obtain the Folium Folium extract; , amantadine hydrochloride, caffeine, chlorpheniramine, and artificial bezoar a...
Embodiment 3
[0080] (1) Prescription: 250g of aspirin, 100g of amantadine hydrochloride, 15g of caffeine, 2g of chlorpheniramine, 10g of artificial bezoar, 500g of honeysuckle, and 500g of Daqingye.
[0081] (2) preparation of main ingredient: get honeysuckle medicinal material, pulverize, cross 40 mesh sieves, add 75% hydrochloric acid ethanol solution of 6 times amount pH3. Minutes, oscillation frequency 35kHz, extraction 2 times, combined extracts, filtered, filtrate under 50 ℃ under reduced pressure to recover ethanol, dried, crushed to obtain honeysuckle extract; 6 times the amount of 85% ethanol reflux extraction twice, each time for 1 hour, combined extracts, filtered, the filtrate was decompressed to recover ethanol, dried, crushed to obtain the Folium Folium extract; Mix with aspirin, amantadine hydrochloride, caffeine, chlorpheniramine, and artificial bezoar to obtain 459 g of the main drug.
[0082] (3) The preparation prescription is:
[0083] Main drug 459g
[0084] Cross-l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com