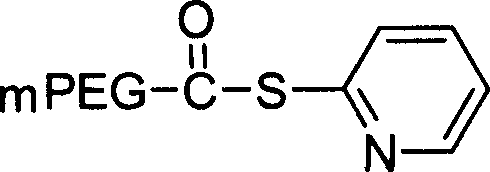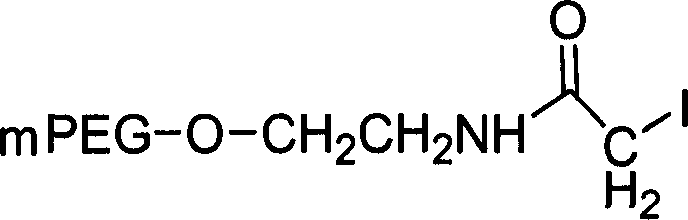Antagonists of the bradykinin b1 receptor
A peptide antagonist, bradykinin technology, applied in the direction of pancreatic kinin/bradykinin, anti-inflammatory agents, specific peptides, etc., can solve problems such as intolerance to substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0288] Example 1: Synthesis and purification of B1 receptor peptide antagonists and PEG-coupled B1 receptor peptide antagonists
[0289] The various peptides of the invention are synthesized using synthetic techniques well known in the art. A preferred method of synthesizing the various peptides of the invention utilizes the activation of carbodiimides using the FMOC method, as described below.
[0290] Part 1: Addition of dissolved Fmoc-amino acids to resins using carbodiimide chemistry.
[0291] Fmoc-amino acids (3-4 equivalents) were dissolved into dry DCM / NMP mixture (NMP or DMF was used to aid complete dissolution). N-Hydroxybenzotriazole (HOBt, same as amino acid equivalent) dissolved in NMP was added to the amino acid solution. N,N'-dicyclohexyl-carbodiimide (DCC, same as amino acid equivalent) dissolved in DCM was added to the amino acid solution. The solution was mixed for about 20 minutes. The activated acid solution is then added to the resin (if necessary, the ...
Embodiment 2
[0347] Example 2: Synthesis and purification of PEG-conjugated B1 receptor peptide antagonists using PEG thioesters.
[0348] in 50mM NaHPO 4 , 5 mM EDTA, pH 7, mix a cysteine-containing peptide with PEG-maleimide (2.5-5 mg / ml peptide and react stoichiometrically with a 1.2-fold molar excess of maleimide:thiol) reaction to prepare PEG-conjugated peptides. The reaction was stirred at room temperature (20-25°C) for 18-26 hours. As soon as the stirring is finished, the reaction is terminated with a 10-fold molar excess of β-mercaptoethanol (β-ME):maleimide, and stirred at room temperature for another 30-60 minutes. The reaction mixture was purified by method A as described in the above examples.
Embodiment 3
[0349] Example 3: Synthesis and purification of PEG-conjugated B1 receptor peptide antagonists using PEG thioester or iodoacetate.
[0350] in 50mM NaHPO 4 , 5 mM EDTA, pH 7, react a peptide containing an N-terminal cysteine with PEG-OPTE (o-pyridylthioester) (2.5-5 mg / ml peptide and a stoichiometric excess of 1.2-fold molar activated PEG: peptide) reaction to prepare PEG-conjugated peptides. The reaction was stirred at room temperature (20-25°C) for 18-26 hours. Once the stirring is over, the reaction is terminated with a 10-fold molar excess of cysteine: excess PEG reagent, and stirred at room temperature for another 30-60 minutes. The reaction was purified using Method A described in Example 1 above.
[0351] Alternatively, conjugates can be formed using PEG-iodoacetamide as described above, where the PEG moieties are linked by thioether linkages (Scheme 3). In this case, 1.5 molar equivalents of the activated PEG reactant were used, the reaction time was increased to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com