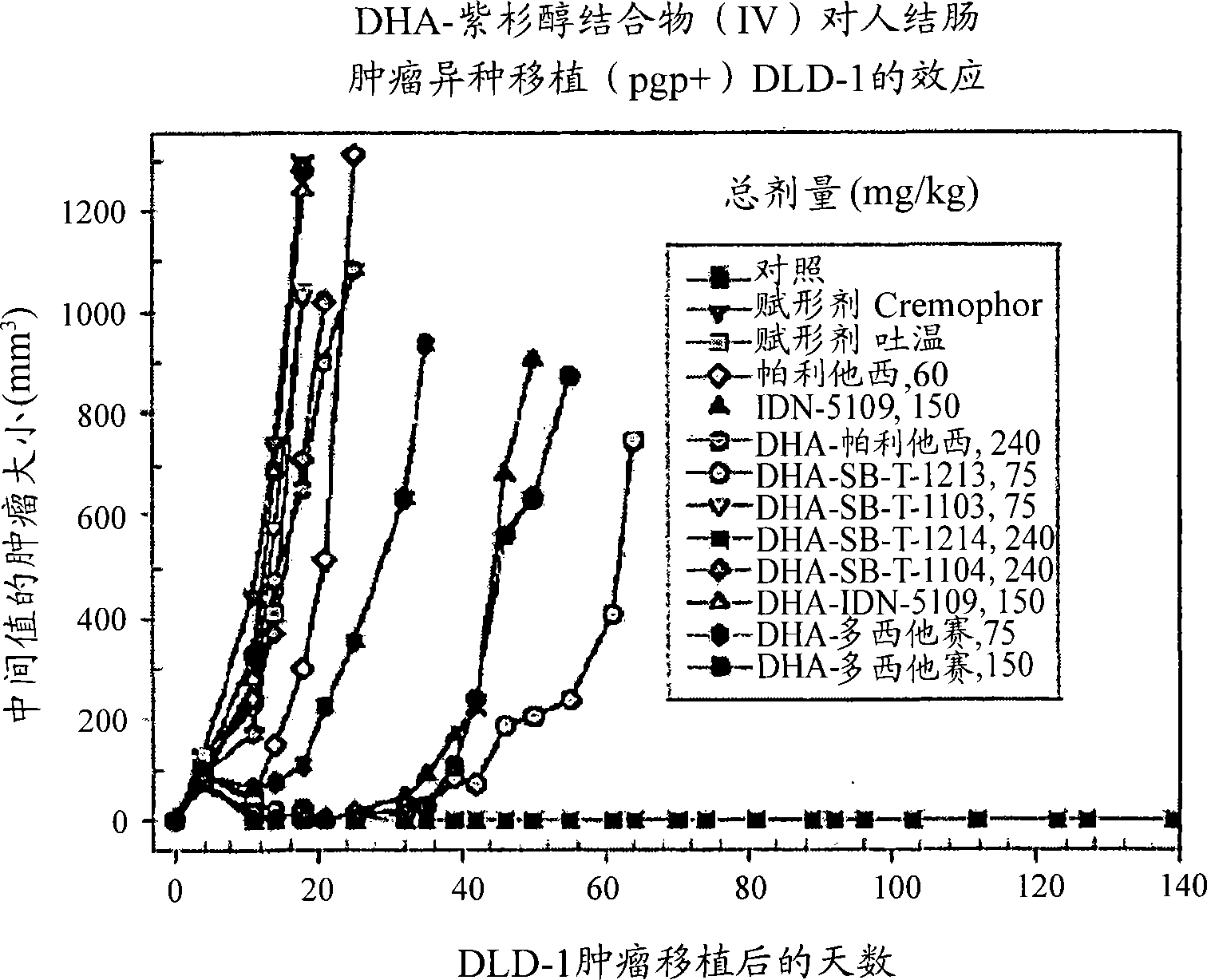
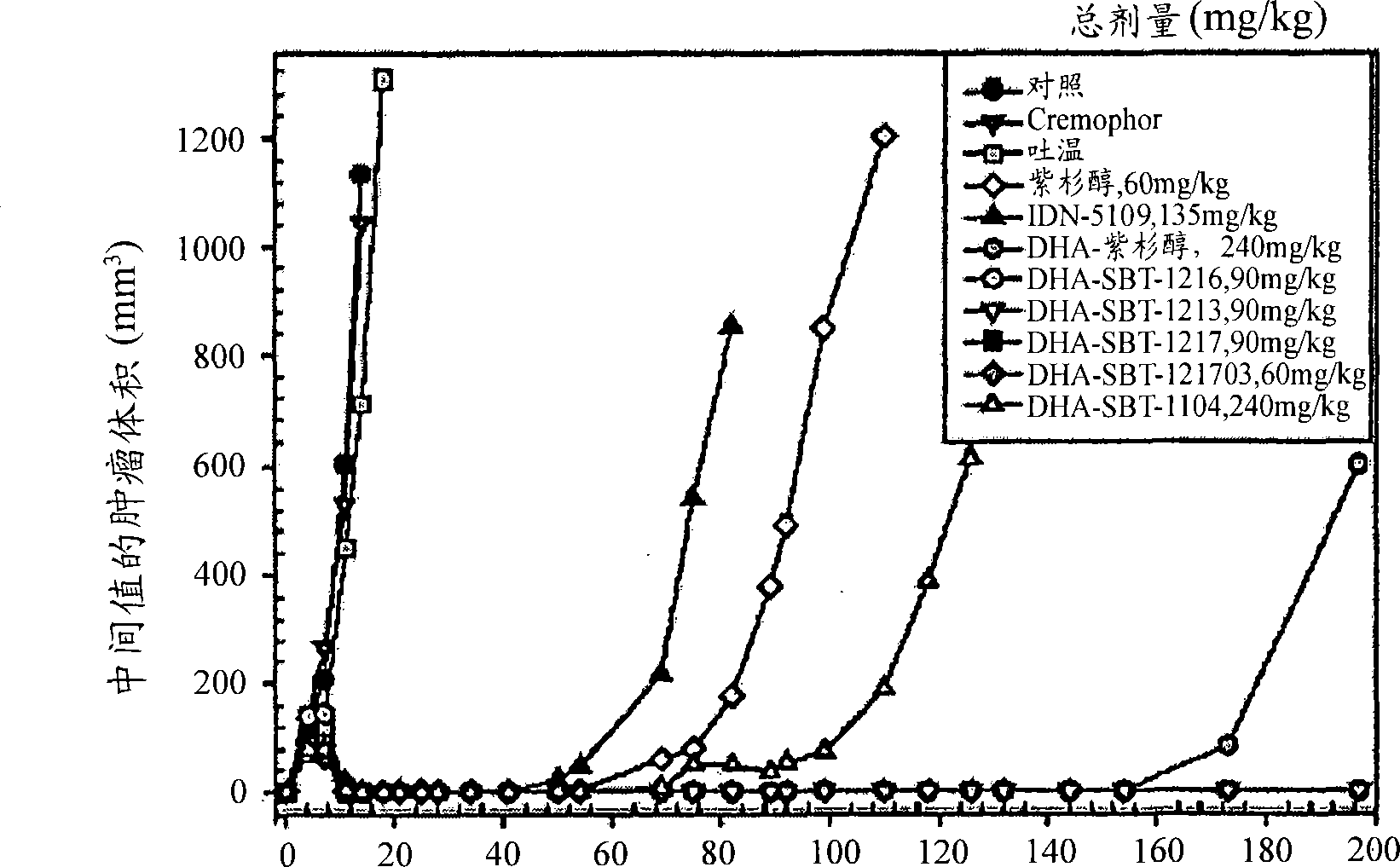
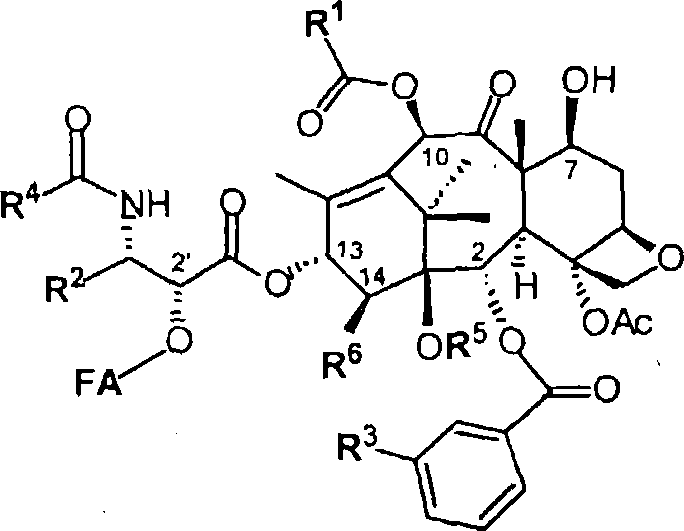
Taxoid-fatty acid conjugates and pharmaceutical compositions thereof
A technology of paclitaxel and conjugates, which can be used in drug combinations, active ingredients of heterocyclic compounds, anti-tumor drugs, etc., and can solve the problems of non-specific drugs for tumors and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] 2'-Docosahexaenoyl-3'-desphenyl-3'-(2-methyl-2-propyl)-10-(methoxycarbonyl)docetaxel (DHA-SB-T -1107):
[0088] 3'-Desphenyl-10-(methoxycarbonyl)-3'-(2-methyl-2-propyl)-2'-eco Add 4-dimethylaminopyridine (9 mg; 75 μmol), 1,3-dicyclohexylcarbodiimide to a solution of docetaxel (SB-T-1107) (63.9 mg; 75 μmol) (19mg, 150μmol), and DHA (27mg; 83μmol). The reaction mixture was stirred at room temperature for 1 h. After diluting with dichloromethane, the reaction mixture was washed with 5% hydrochloric acid, water, and brine. The organic layer was dried over anhydrous magnesium sulfate, and concentrated under vacuum. The crude product was purified by chromatography on silica gel (ethyl acetate / hexane = 1 / 3 to 1 / 1) to give 78.5 mg (90% yield) of DHA-SB-T as a white solid -1107: melting point m.p.102-105°C, [α] D 22 -45.0 (c1.0, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ0.96(m, 9H), 1.14(s, 3H), 1.22(s, 3H), 1.30(s, 9H), 1.67(m, 3H), 1.69(s, 3H), 1.88(m, 1H ), 1.96(s, 3H), ...
Embodiment 2-9
[0090] Other DHA-paclitaxel was synthesized in the same manner as described in Example 1 for the synthesis of DHA-SB-T-1107. Characterization data for these DHA-paclitaxels are shown below.
Embodiment 2
[0092] 2’-Docosahexaenoyl-3’-desphenyl-3’-(2-methylpropyl)-10-propionyl docetaxel (DHA-SB-T-1103):
[0093] 75% yield; white solid; m.p. 94-98°C, [α] D 22 -37.9 (c 1.08, CHCl 3 ); 1 H NMR (400MHz, CDCl 3)δ0.97(m, 9H), 1.13(s, 3H), 1.22-1.27(m, 6H), 1.31(s, 9H), 1.56(s, 3H), 1.67(s, 3H), 1.90(m , 1H), 1.94(s, 3H), 2.08(m, 2H), 2.39(s, 3H), 2.40(m, 2H), 2.46-2.60(m, 7H), 2.85(m, 10H), 3.82( d, J=7.0Hz, 1H), 4.20(d, J=8.4Hz, 1H), 4.30(d, J=8.4Hz, 1H), 4.35(m, 1H), 4.46(dd, J=10.2, 6.7 Hz, 1H), 4.60(d, J=10.4Hz, 1H), 4.92(d, J=2.4Hz, 1H), 4.98(d, J=7.6Hz, 1H), 5.40(m, 12H), 5.67( d, J=7.6Hz, 1H), 6.23(m, 1H), 6.31(s, 1H), 7.48(t, J=7.6Hz, 2H), 7.60(t, J=7.6Hz, 1H), 8.12( d, J=7.6Hz, 2H); 13 C NMR (400MHz, CDCl 3 )δ9.0, 9.6, 14.1, 14.3, 14.8, 20.5, 21.9, 22.2, 22.4, 22.5, 22.6, 23.2, 24.5, 25.5, 25.6, 26.6, 27.5, 28.1, 28.3, 33.7, 35.5, 41.3, 43.2, 45.6 , 48.9, 58.8, 71.5, 72.2, 74.4, 75.2, 75.5, 76.4, 77.3, 79.3, 79.8, 81.0, 84.4, 127.0, 127.5, 127.8, 127.9, 128.0, 128.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com