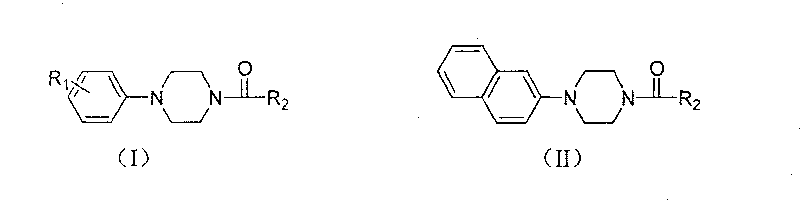
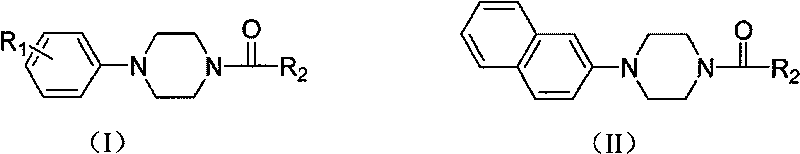
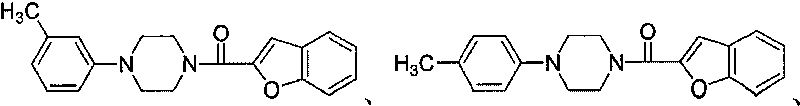Aryl formyl piperazine compound and its preparing method and use in medicine production
A technique for aroylpiperazines and compounds, which is applied in the field of preparation of the aroylpiperazines, can solve problems such as weakening effects, and achieve the effects of mild reaction conditions, strong antiarrhythmic activity, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: 2-thienyl-[1-(4-(3-methyl)phenyl)piperazine]methanone hydrochloride
[0072]
[0073] (1) Preparation of 1-(3-methylphenyl)piperazine hydrochloride: add m-toluidine 1.07g (0.01mol) in nitrogen mustard 1.785g (0.01mol), anhydrous K 2 CO 3 0.69g (0.005mol) and 20ml of n-butanol, stirred and refluxed for 48 hours. Concentrate, refrigerate, precipitate a solid, filter it out, and recrystallize the solid from absolute ethanol to obtain 1.05 g of white flaky crystals, m.p.: 127-129°C (dec), yield: 49.41%.
[0074] (2) Preparation of the title compound: Add 10ml of chloroform to 0.64g (0.005mol) of thiophene-2-carboxylic acid, add 2.38g (0.02mol) of SOCl dropwise 2 10ml of chloroform solution, reflux for 1 hour. Concentrate to dryness and dissolve in 15ml of chloroform to obtain a solution of acid chloride in chloroform for use.
[0075] Add 10ml of chloroform to 0.53g (0.0025mol) of 1-(3-methyl)phenylpiperazine hydrochloride, stir at room temperature, slowly...
Embodiment 2
[0079] Example 2: 2-benzofuryl-[1-(4-(3-methyl)phenyl)piperazine]methanone
[0080]
[0081] Prepared according to the method of Example 1, substituting benzofurancarboxylic acid for thiophene-2-carboxylic acid in step (2) to obtain 0.29 g of the title compound as pale yellow flaky crystals, m.p.: 167-168°C, yield: 45.31%.
[0082] IR(KBr), cm -1 : 2823, 1612, 1443, 1432, 1248, 953, 773, 748, 689
[0083] EI-MS: m / z320: M m / z146:
[0084] 1 H-NMR (CDCl 3 ), δ: 7.67 (dd, 1H, ), 7.54(dd, 1H, Ar-H), 7.42(t, 1H, Ar-H), 7.35(s, 1H, Ar-H), 7.31(t, 1H, Ar-H), 7.18(t, 1H, ), 6.75(t, 3H, ), 4.01(s, 4H, ), 3.27(d, 4H, ), 2.33(s, 3H, -C H 3 )ppm.
Embodiment 3
[0085] Example 3: 3-Chlorophenyl-[1-(4-(3-methyl)phenyl)piperazine]methanone hydrochloride
[0086]
[0087] According to the method of Example 1, m-chlorobenzoic acid was used instead of thiophene-2-carboxylic acid in step (2) to obtain 0.30 g of the title compound as a white loose solid. m.p.: 199-200°C, yield: 34.19%.
[0088] IR(KBr), cm -1 : 2998, 2328, 1655, 1427, 1256, 1110, 1035, 926, 767
[0089] EI-MS: m / z314: M m / z146:
[0090] 1 H-NMR (CDCl 3 ), δ: 7.68(s, 1H, Ar-H), 7.60(dd, 1H, Ar-H), 7.45-7.49(m, 2H, Ar-H), 7.38-7.43(m, 2H, Ar-H ), 7.33-7.38(m, 1H, Ar-H), 7.28-7.31(d, 1H, Ar-H), 4.39(s, 4H, ), 3.49(d, 4H, ), 2.42(s, 3H, - CH 3 )ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com