Benzofuran compound, preparation method thereof and application of benzofuran compound in preparation of antiarrhythmic drugs
A technology of phenylfuran and compounds, applied in the field of drug synthesis, can solve the problem of weakened antiarrhythmic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
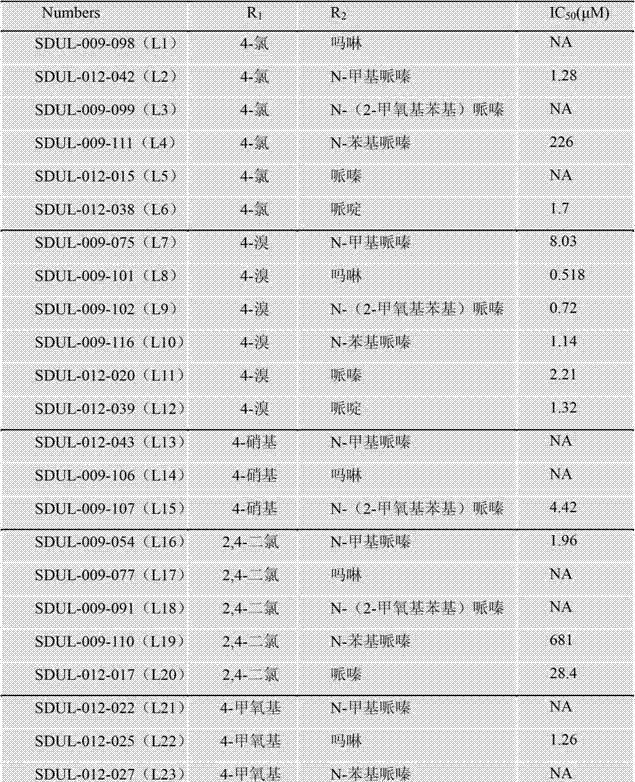
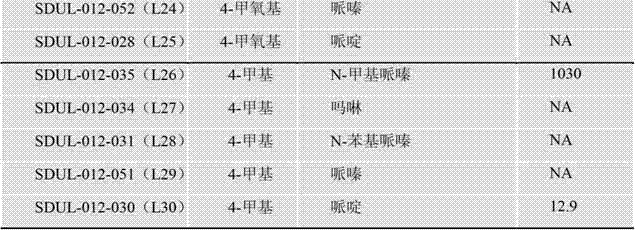
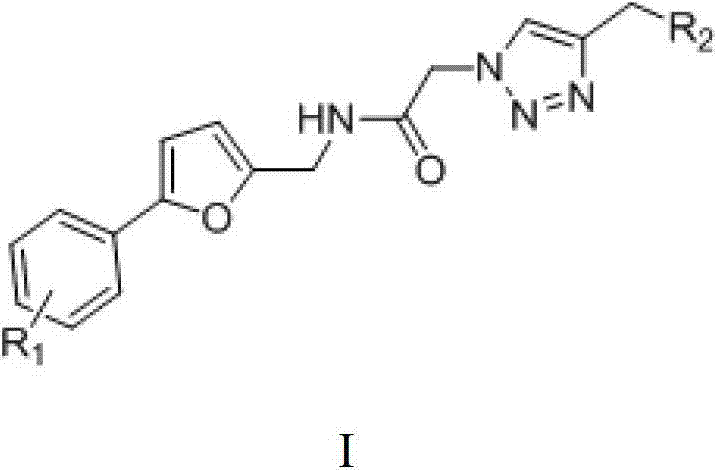
[0073] Example 1: N-[[5-(4-chlorophenyl)furan-2-yl]methyl]-2-[4-[(4-methylpiperazin-1-yl)methyl]-1H -1,2,3-Triazol-1-yl]acetamide
[0074](1) Add 20mL of distilled water and 14mL of concentrated hydrochloric acid to p-chloroaniline (2.55g, 20mmol), it turns white and turbid, heat to 60°C until completely dissolved, cool to 0°C in an ice-salt bath, slowly add sodium nitrite solution dropwise (1.38g dissolved in 10mL distilled water), after dropping, continue to react for 20min, add furfurylamine (2.19g, 30mmol) and copper chloride (0.54g, 4mmol), the reaction solution is green, stir overnight, a brown solid is precipitated, and suction filtered , to obtain a reddish-brown solid, the filter cake was washed successively with a small amount of water and ethyl acetate to obtain an off-white solid, the solid was decolorized and recrystallized with methanol / ethanol activated carbon, and dried in vacuo to obtain a white solid intermediate 5-(4-chlorophenyl) - 0.88g of 2-aminomethylfu...
Embodiment 2
[0079] Example 2: N-[[5-(4-bromophenyl)furan-2-yl]methyl]-2-(4-morpholinomethyl-1H-1,2,3-triazole- 1-yl)acetamide
[0080] (1) Prepared according to the method of step (1) in Example 1, except that p-bromoaniline (3.44g, 20mmol) and furfurylamine (2.91g, 30mmol) were reacted to obtain a white solid 5-(4-bromophenyl) -2-Aminomethylfuran 1.18g, the yield is 20.4%, 1 H-NMR(600MHz,DMSO-d6):δ=8.61(s,3H),7.66(m,4H),7.03(d,J=3.6Hz,1H),6.66(d,J=3.6Hz,1H) ,4.13(d,J=3.6Hz,2H);ESI-MS:[M-NH 2 ] + : 235.1, 237.1.
[0081] (2) Prepared according to the method of step (2) in Example 1, except that 5-(4-bromophenyl)-2-aminomethylfuran (2.16g, 8.5mmol) and chloroacetyl chloride (1.44g, 12.75mmol) to obtain 0.65g of yellow solid 2-chloro-N-[[5-(4-bromophenyl)-2-furyl]methyl]-acetamide, the yield is 58%, m.p:131-133 °C, 1 H-NMR (600MHz, DMSO-d6): δ=8.77(t,1H),7.62(m,4H),6.95(d,J=3Hz,1H),6.40(d,J=3Hz,1H),4.36 (d,J=5.4Hz,2H),4.12(s,2H);ESI-MS:[M+H] + :327.7;[M+NH 4 ] + :344.7, 346.8.
...
Embodiment 3
[0085] Example 3: N-[[5-(4-nitrophenyl)furan-2-yl]methyl]-2-[4-[[4-(2-methoxyphenyl)piperazine-1 -yl]methyl]-1H-1,2,3-triazol-1-yl]acetamide
[0086] (1) Prepared according to the method of step (1) in Example 1, except that p-nitroaniline (2.76g, 20mmol) and furfurylamine (2.91g, 30mmol) were reacted to obtain a yellow solid 5-(4-nitrobenzene Base)-2-aminomethylfuran 1.10g, yield is 21.6%. 1H-NMR(600MHz,DMSO-d6):δ=8.65(s,3H),8.31(m,2H),8.00(m,2H),7.32(d,J=3Hz1H),6.76(d,J=3Hz ,1H), 4.19(s,2H); ESI-MS: [M-NH2]+: 202.3.
[0087] (2) Prepared according to the method of step (2) in Example 1, except that 5-(4-nitrophenyl)-2-aminomethylfuran (0.98g, 3.4mmol) and chloroacetyl chloride (0.77g , 6.8mmol) reacted to obtain a yellow solid 1.56g, the yield was 63%, m.p:144-146°C, 1 H-NMR (600MHz, CDCl 3 ):δ=8.24(d,J=9Hz,2H),7.76(d,J=8.4Hz,2H),6.96(s,1H),6.82(d,J3.6Hz,1H),6.43(d,J =3.6Hz,1H),4.58(d,J=5.4Hz,2H),4.12(s,2H);ESI-MS:[M+H] + :295.4,[M+Na] + :317.3.
[0088] (3) Prepared ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com