Method for filtering out natural products anti AIDS through activity guidence
A natural product, active technology, applied in biochemical equipment and methods, microbial determination/inspection, biological testing, etc., can solve the problems of inability to use crude extracts to screen, difficult to widely use, complex operation, etc., and achieve convenient and fast application. , the effect of wide application range and wide application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
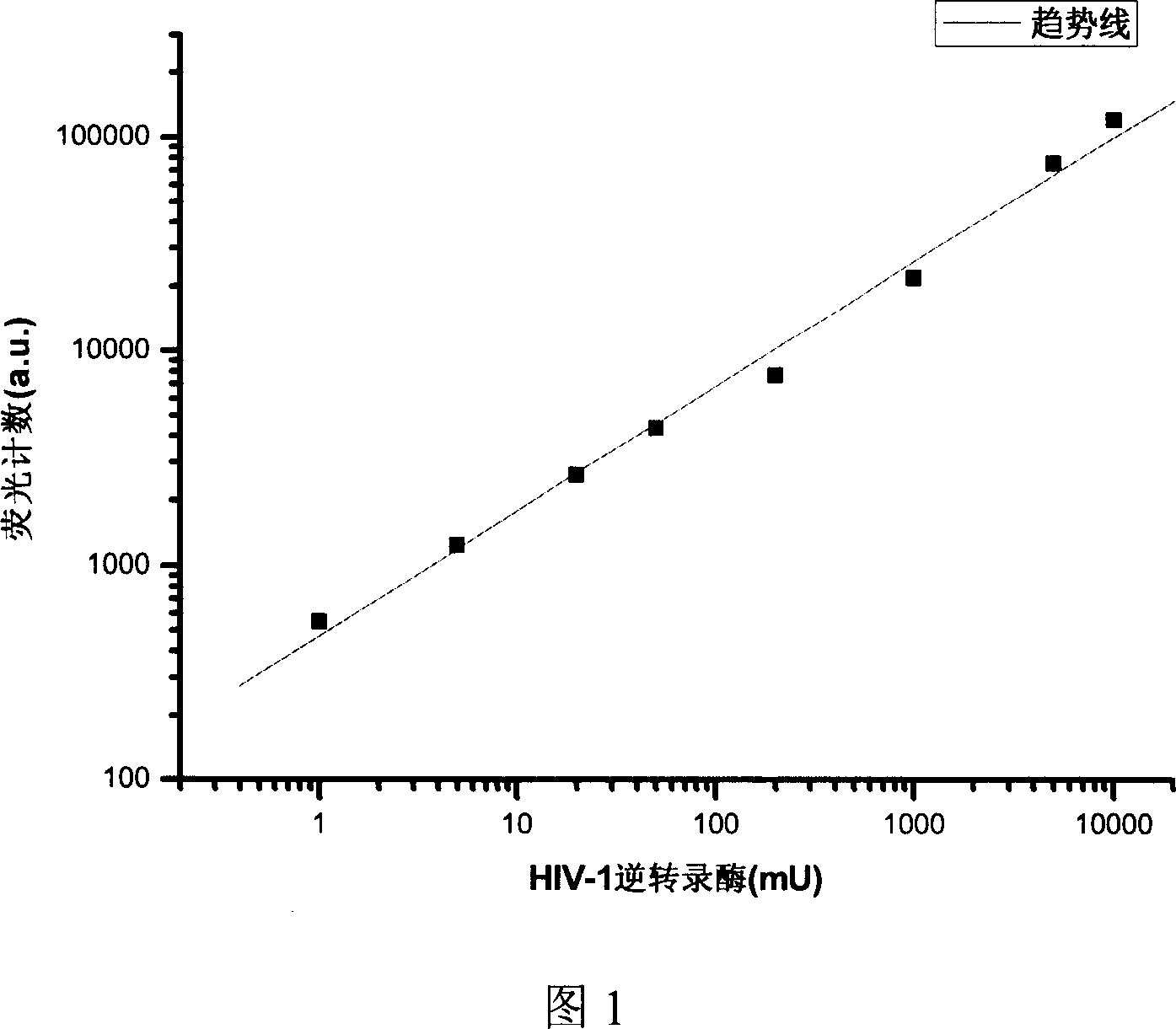
[0033] Dilute anti-DIG in PBS buffer (137mM NaCl, 2.7mM KCl, 10mM NaCl 2 HPO 4 , 2mM KH 2 PO 4), prepare a coating solution with a final concentration of 8 mg / l. Add 60 μl of coating solution to each well, and coat for 24 hours. Wash the plate 3 times with Buffer A (50mM Tris-HCl, pH 7.8, 0.15M NaCl, 0.05% Tween-20) and once with Wash Buffer B (50mM Tris-HCl, pH 7.8, 0.15M NaCl) . Each well was blocked with 75 μl of blocking solution (1% BSA, 50 mM Tris-HCl, pH 7.4, 0.15 M NaCl) for 1 hour. Wash the plate 3 times with buffer A and once with buffer B. Prepare the reaction mixture (0.75A 260nm / ml template / primer[poly[A] / poly[dT] 15 ], 10 μM dTTP, 5 μM biotin-dUTP, 5 μM DIG-dUTP, 45.8 mM Tris, 265.8 mM KCl, 27.5 mM MgCl 2 , 9.17mM DTT), HIV-1 RT was diluted in lysis buffer (50mM Tris-HCl, pH 7.8, 80mM KCl, 2.5mM DTT, 0.75mM EDTA, 0.5% TritonX-100), and the final concentration of preparation was 0.05~ 500mU / μl HIV-1 RT solution, 20μl reaction mixture, 20μl lysis buffer ...
Embodiment 2
[0036] Anti-DIG was diluted in PBS buffer solution to prepare a coating solution with a final concentration of 8mg / l. Add 60 μl of coating solution to each well, and coat for 24 hours. Wash the plate 3 times with buffer A and once with buffer B. Each well was blocked with 75 μl blocking solution for 1 hour. Wash the plate 3 times with Wash Buffer A and once with Wash Buffer B. Prepare the reaction mixture (0.75A 260nm / ml template / primer[poly[A] / poly[dT] 15 ], 10 μM dTTP, 5 μM biotin-dUTP, 5 μM DIG-dUTP, 45.8 mM Tris, 265.8 mM KCl, 27.5 mM MgCl 2 , 9.17mM DTT), HIV-1 RT was diluted in the lysis buffer, and the final concentration of preparation was 50mU / μl HIV-1 RT solution, and a certain amount of Efavirenz was dissolved in the lysis buffer to prepare the Efavirenz solution, and 20 μl of the reaction mixture , 20 μl Efavirenz solution and 20 μl HIV-1 RT solution were added to the PCR tube, and the reverse transcription reaction was performed on a PCR instrument at 37°C. ...
Embodiment 3
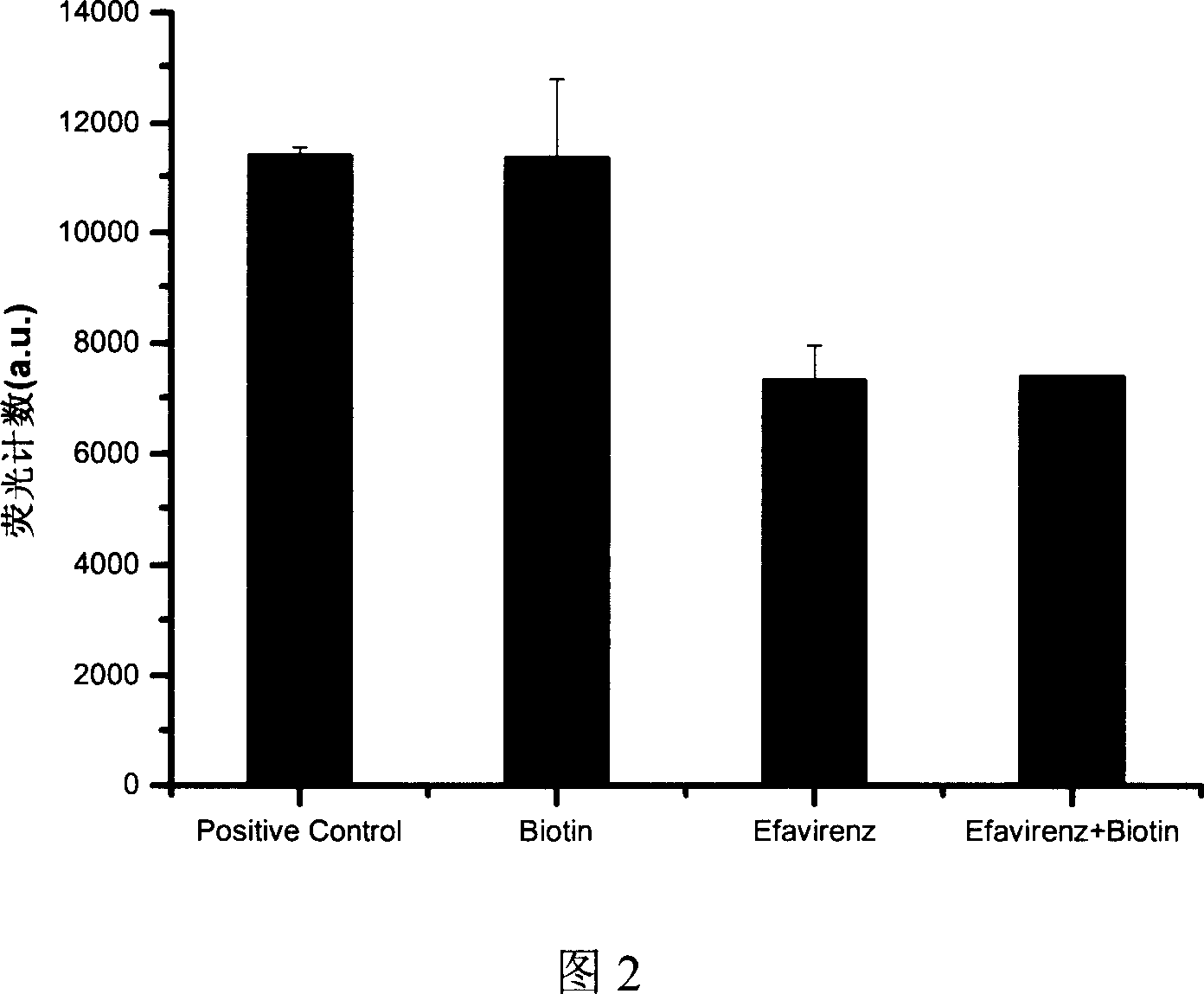
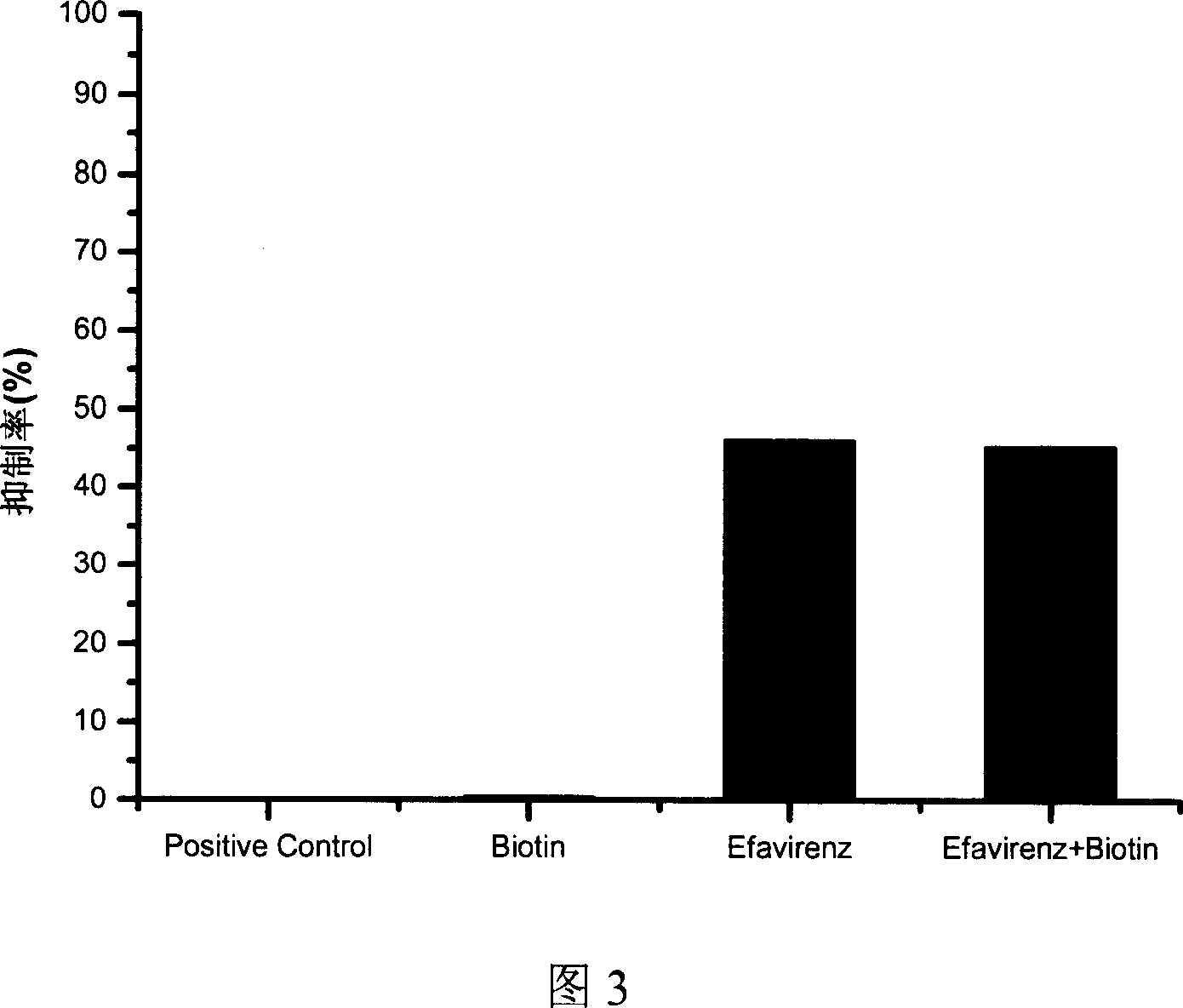
[0040] Dissolve Efavirenz in lysis buffer to a final concentration of 250 ng / ml. Dissolve biotin in lysis buffer to a final concentration of 2 μg / ml. Efavirenz and biotin were co-dissolved in lysis buffer to a final concentration of 250 ng / ml Efavirenz and 2 μg / ml biotin. These 3 solutions and the positive control were used for method testing, and the results are shown in Figure 2 and Figure 3. The incorporation of biotin has almost no effect on the detection of positive controls and known inhibitors. According to literature reports, the method of using commercial kits can cause an inhibition rate of 77.78% when incorporation of 0.2 μg / ml biotin is detected, which is False positives caused by biotin affecting the method itself. Even when the method is mixed with 10 times the concentration of 2μg / ml biotin, it still has almost no effect on the determination, which shows that this method can effectively avoid the interference caused by endogenous biotin, and can be used for th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com