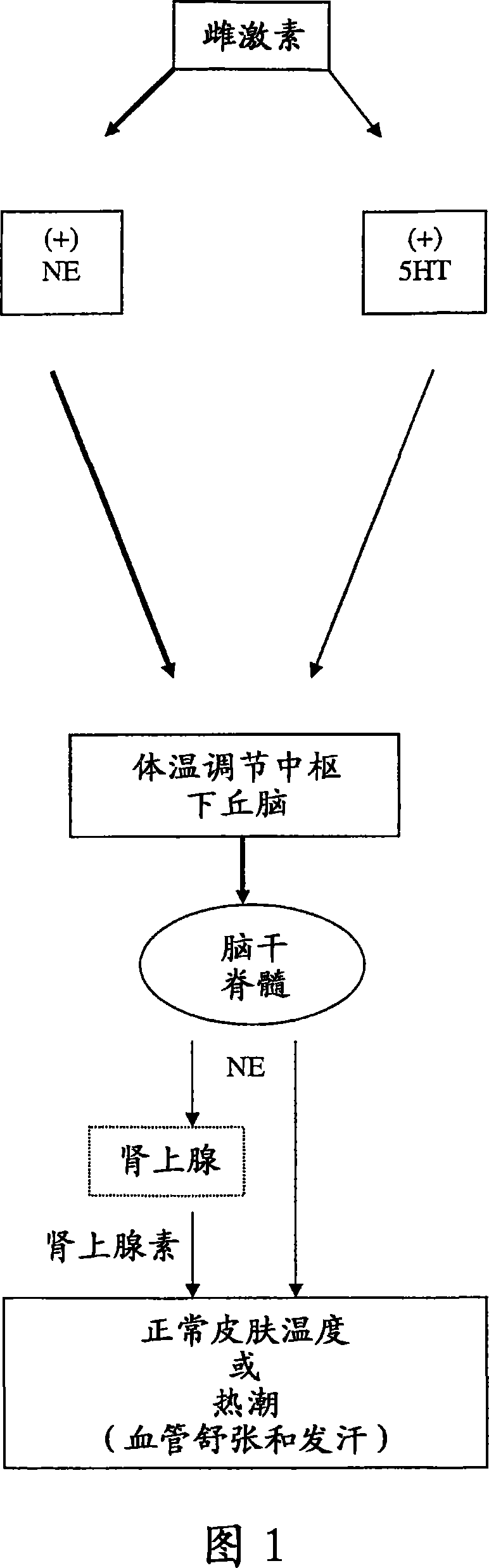
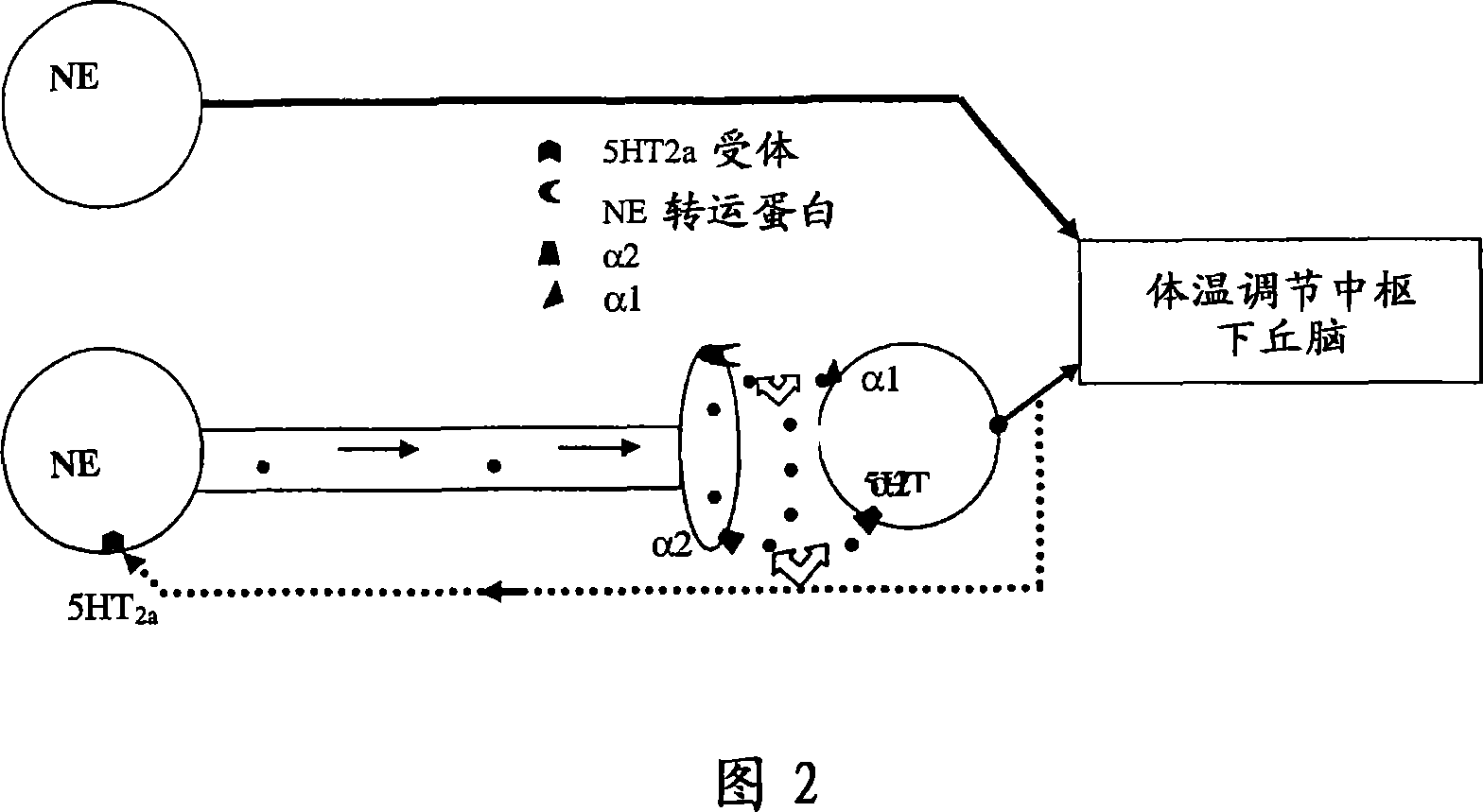
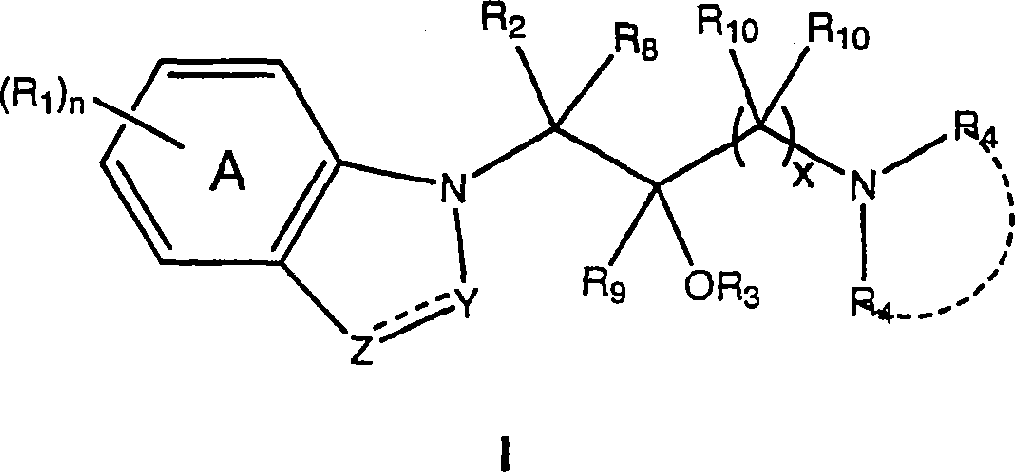
1-(1h-indol-1-yl)-3-(4-methylpiperazin-1-yl)-1-phenyl propan-2-ol derivatives and related compounds as modulators of the norepinephrine(NE) and the serotonine(5-HT) activity and the monoamine reuptake
A compound, R11 technology, applied in the direction of drug combination, organic active ingredients, diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0693] Example 1: (1RS, 2SR)-1-(1H-indol-1-yl)-3-(4-methylpiperazin-1-yl)-1-phenylpropan-2-ol dihydrochloride Salt
[0694]
[0695] Step 1: A mixture of indole (2.34 g, 20 mmol) and crushed solid potassium hydroxide (1.12 g, 20 mmol) was stirred at room temperature under nitrogen atmosphere for 30 minutes. A solution of trans-3-phenylglycidol (3.0 g, 20 mmol) in dimethylsulfoxide (1 mL) was then added and the mixture was stirred at 70° C. for 2 hours until no epoxide remained. The mixture was then cooled and partitioned between water and dichloromethane. The organic layer was separated, washed several times with water, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The crude product was purified via Biotage chromatography (FlasH40i, silica, 10%, 20%, 30% ethyl acetate / hexanes) to give 1.92 g (36%) of (2RS, 3RS)-3-indole-1- Base-3-phenyl-propane-1,2-diol as an oil. 1 HNMR (DMSO): δ3.27 (m, 2H, CH 2 OH), δ4.45(m, 1H, CHOH), δ4.80(t, 1H, CHOH 2...
Embodiment 2
[0698] Example 2: (1RS, 2SR)-1-(5-fluoro-1H-indol-1-yl)-3-(4-methylpiperazin-1-yl)-1-phenylpropane-2- Alcohol dihydrochloride
[0699]
[0700] From 5-fluoroindole and trans-3-phenylglycidol, (2RS, 3RS)-3-(5-fluoro-indol-1-yl)-3 was prepared in a manner similar to Example 1 step 1 -Phenyl-propane-1,2-diol as an oil. MS (ESI) m / z 286 ([M+H] + ).
[0701] Prepare (2RS, 3RS)-Toluene-4-sulfonic acid 3-(5-fluoro-indol-1-yl)-2-hydroxy-3-phenyl-propyl ester. MS (ESI) m / z 440 ([M+H] + ).
[0702] From (2RS, 3RS)-toluene-4-sulfonic acid 3-(5-fluoro-indol-1-yl)-2-hydroxyl-3-phenyl-propyl ester, according to steps similar to Example 1 step 3 Preparation of (1RS, 2SR)-1-(5-fluoro-1H-indol-1-yl)-3-(4-methylpiperazin-1-yl)-1-phenylpropan-2-ol di Hydrochloride.
[0703] MS m / z 368 ([M+H] + ); HRMS: calculated value C 22 h 26 FN 3O +H+, 368.21327; Found (ESI, [M+H]+), 368.213.
Embodiment 3
[0704] Example 3: (1RS, 2SR)-1-(1H-indol-1-yl)-3-morpholin-4-yl-1-phenylpropan-2-ol hydrochloride
[0705]
[0706] From (2RS, 3RS)-toluene-4-sulfonic acid 2-hydroxy-3-indol-1-yl-3-phenylpropyl ester (Example 1, step 3) and morpholine, following a procedure analogous to Example Preparation of (1RS, 2SR)-1-(1H-indol-1-yl)-3-(4-methylpiperazin-1-yl)-1-phenylpropane-2-alcohol salt in step 3 salt.
[0707] MS (ESI) m / z 337 ([M+H] + ); HRMS: calculated value C 21 h 24 N 2 o 2 +H+, 337.19105; Found (ESI, [M+H]+), 337.1909.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com