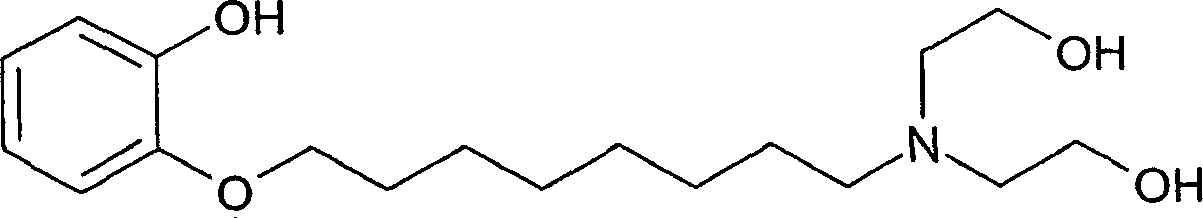
8-(2-hydroxyphenoxy) octyldiethanolamine and salts thereof for delivery of active agents
A technology of octyldiethanolamine and hydroxyphenoxy, applied in the field of 8-(2-hydroxyphenoxy)octyldiethanolamine and its salts for delivery of active agents, which can solve the problem of changing active agents and short shelf life , the system is difficult to manufacture and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: the preparation of compound
[0094] 1a: Preparation of 8-(2-hydroxyphenoxy)octyldiethanolamine) free acid
[0095] The free acid of HPOD was prepared as follows. The free acid of HPOD (i.e., 8-(2-hydroxyphenoxy)octyldiethanolamine) was prepared by the method described in Example 1 of International Publication No. WO 00 / 59863 with suitable starting materials, This international publication is incorporated herein by reference in its entirety.
[0096] The free acid of HPOD was prepared by preparing a solution of 27.5ml (31.4g, 157mmol) of 2-benzyloxyphenol, 80.0ml (118.82g, 434mmol) of 1,8-dibromooctane and 100ml of ethanol. This was treated with 23.18 g (168 mmol) of potassium carbonate and heated to reflux for 5.5 hours. The cooled reaction mixture was stirred at 25°C for 20 hours, filtered and concentrated. The residue was diluted with 100 mL 2:1 hexane / ethyl acetate and decolorized with charcoal. The solution was concentrated. The residue was puri...
Embodiment 2
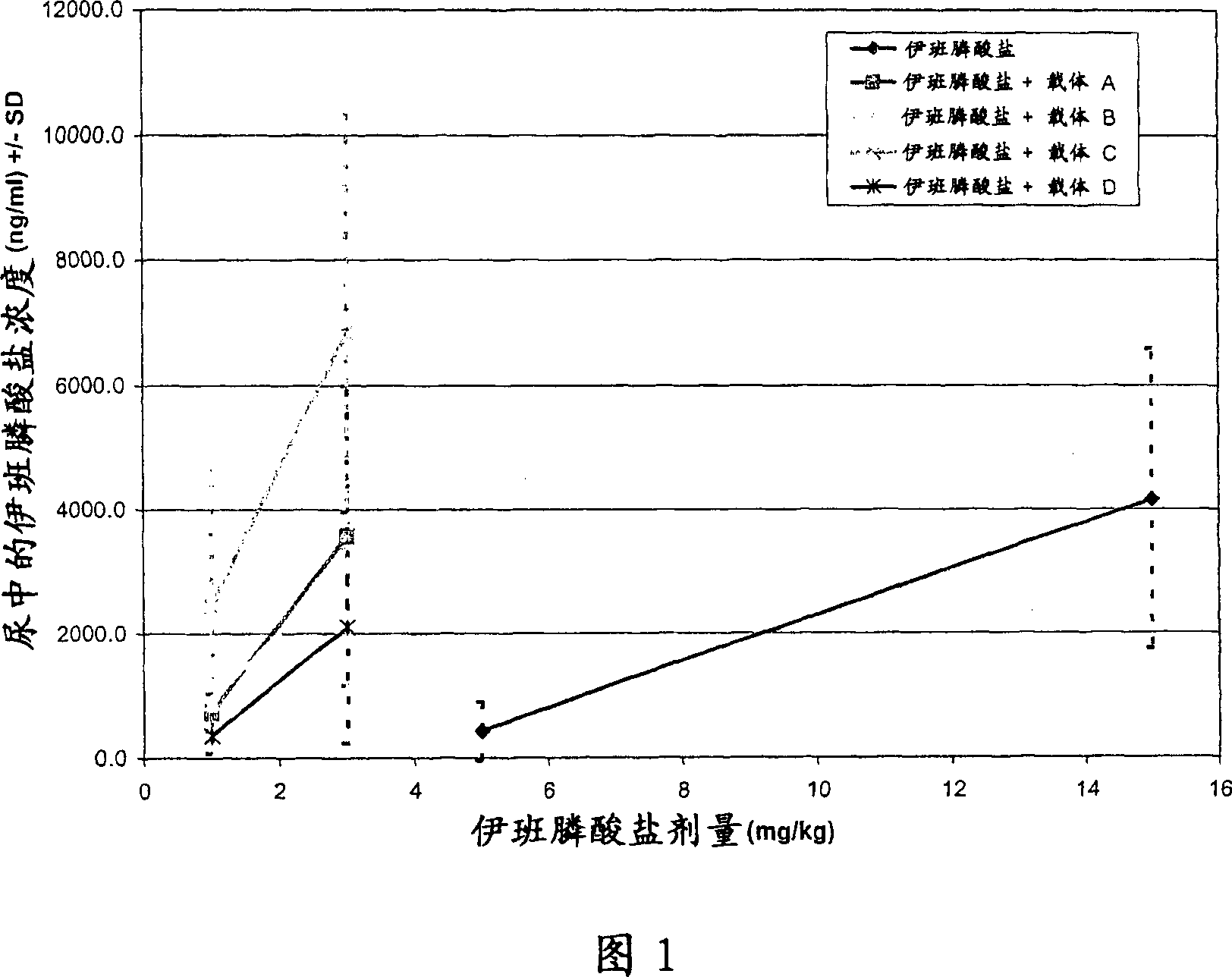
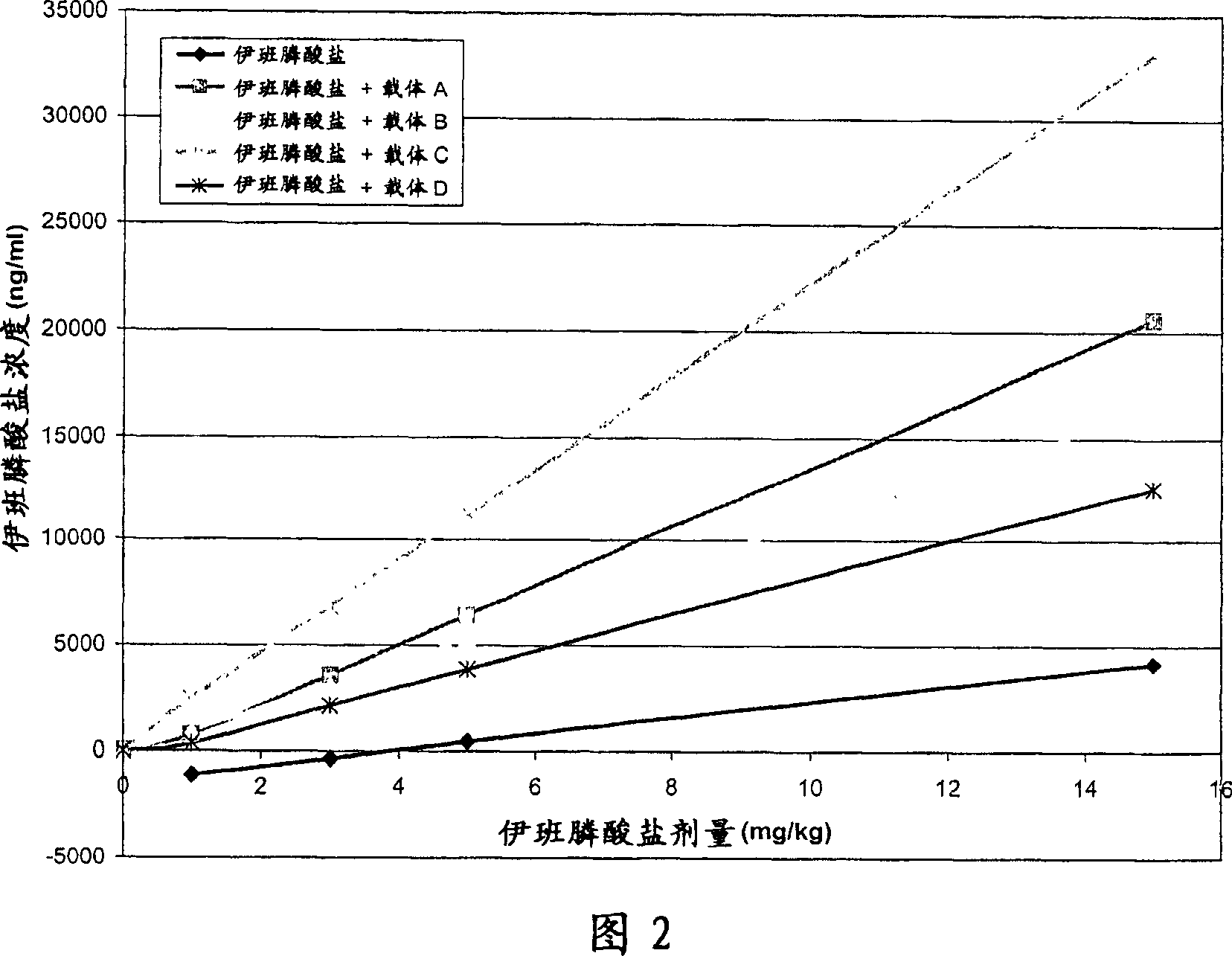
[0105] Example 2: Oral absorption of ibandronate
[0106] overview
[0107] Oral absorption studies in rats were performed to evaluate the delivery of the bisphosphonate ibandronate when co-administered with the delivery agent compounds of the present invention. Sprague-Dawley rats were administered ibandronate alone or in combination with delivery agent compounds by oral gavage. Urine samples were collected up to 16 hours post-dose for ibandronate quantification. The data show that the levels of ibandronate obtained after co-administration with the delivery agent compounds of the present invention by the oral route are much higher than those obtained with oral administration of ibandronate alone. Formulations containing HPOD mesylate resulted in 5.7-fold higher urinary ibandronate levels and 5-fold lower doses compared to ibandronate alone.
[0108] Materials and methods
[0109] Dosing and Dosing Solutions
[0110] For four delivery agents: monosodium N-[8-(2-hydroxyben...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com