Preparation method of active carboxypeptidase B
A carboxypeptidase, active technology, applied in the field of preparation of active carboxypeptidase B, can solve problems such as complicated steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
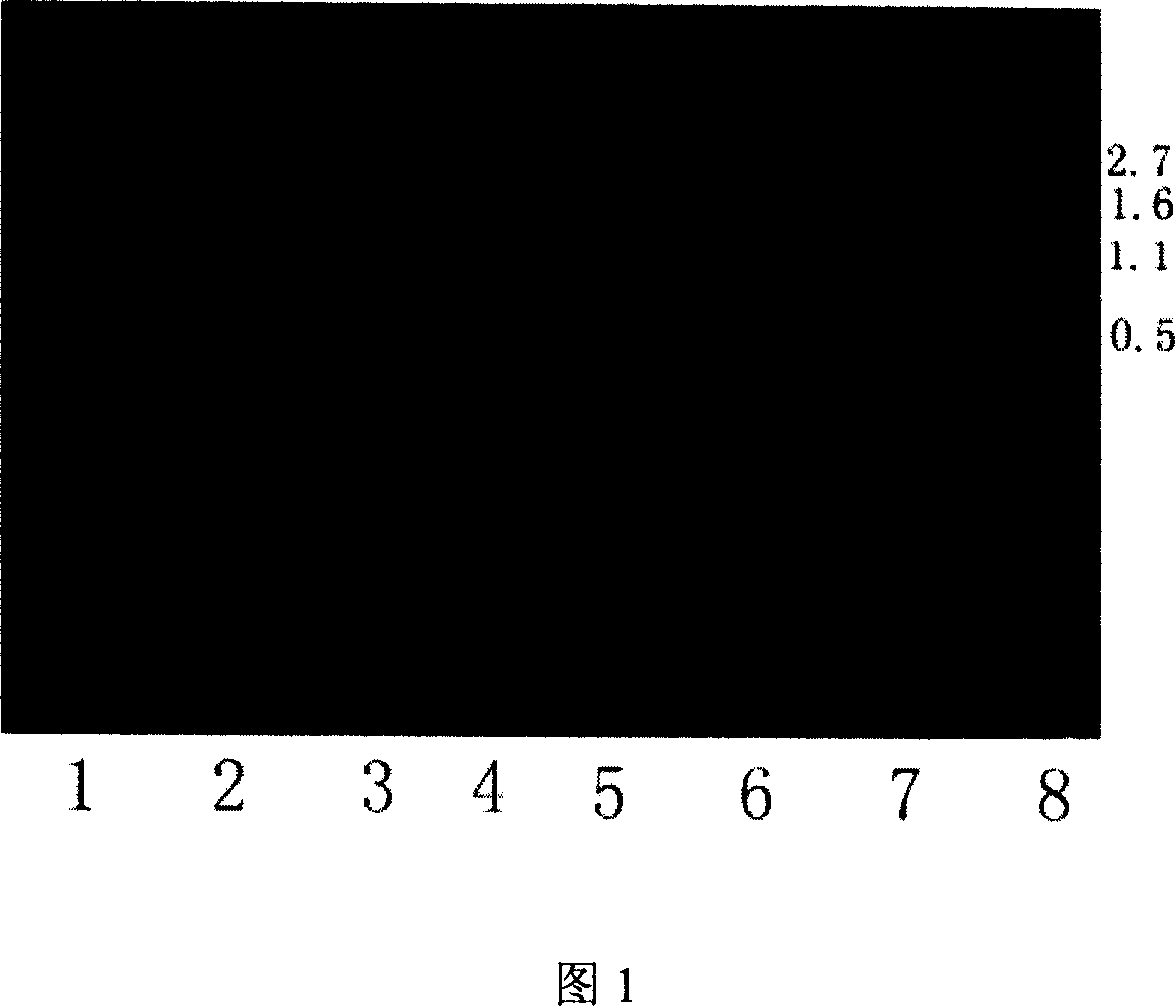
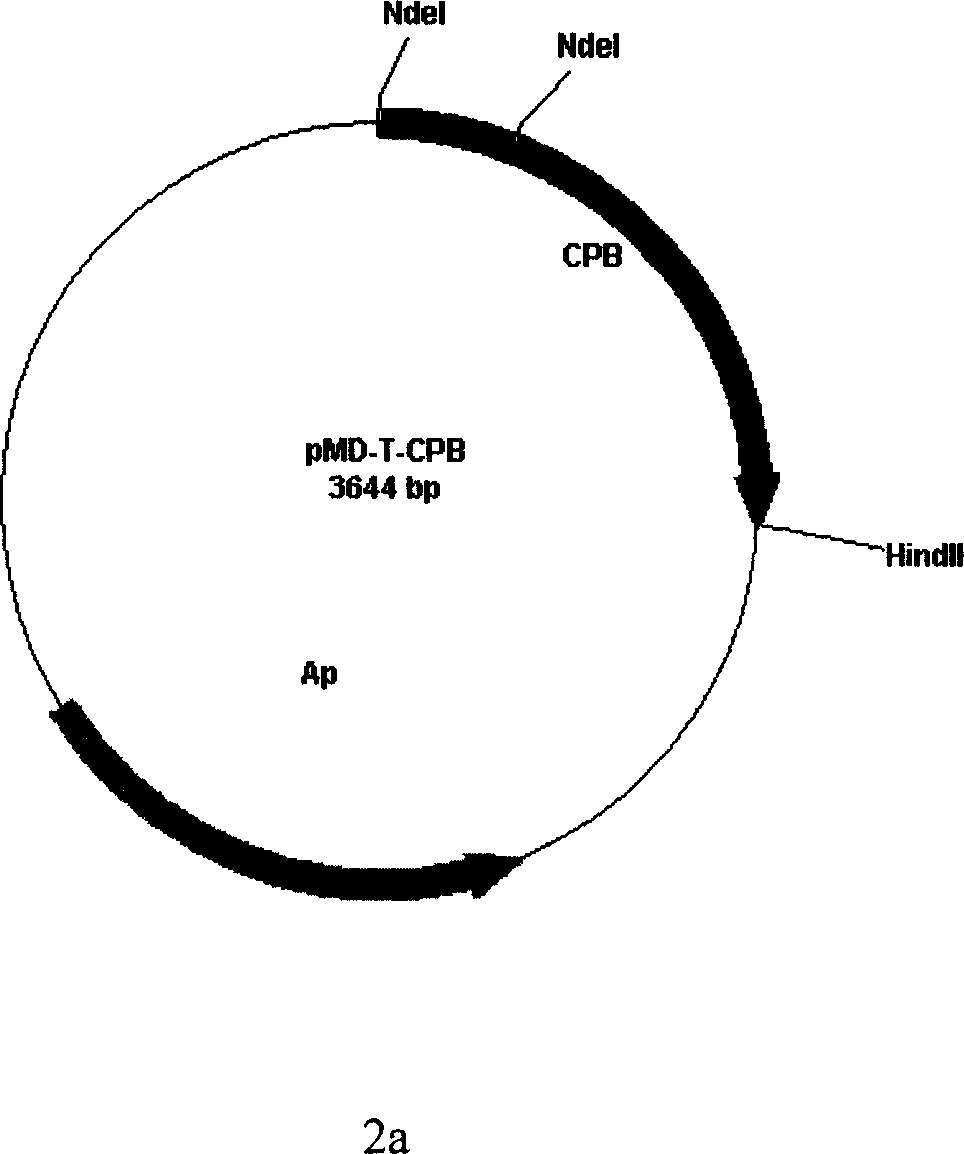
[0044] From the LB solid culture plate (LB culture solution, add agar powder to a concentration of 1.5 (wt%)) to pick a recombinant single colony in 5ml LB liquid culture solution containing 100 μg / ml ampicillin (1% (wt%) tryptone, 0.5 %(wt%)yeast extract, 0.5%(wt%)NaCl, pH7.0), 37°C, shake the bacteria overnight; the overnight bacteria were inoculated at 1% (v / v) in 250ml LB containing 100μg / ml ampicillin culture medium at 37°C until OD 600 0.5, 0.5mmol / L IPTG induction, lower the temperature to 18°C, continue to cultivate for 5 hours, harvest the bacteria, centrifuge and discard the supernatant, suspend the bacteria sediment with 5-100mmol / L pH8.0 TE buffer, ultrasonically break the bacteria body, centrifuged to take the supernatant, and measured the enzyme activity in the supernatant, the activity was 0.15u / ml, adding ZnCl 2 Make the final concentration of 0.01mmol / L in the supernatant, shake gently and mix well, and after standing for 30min, measure the activity of CPB, w...
Embodiment 2
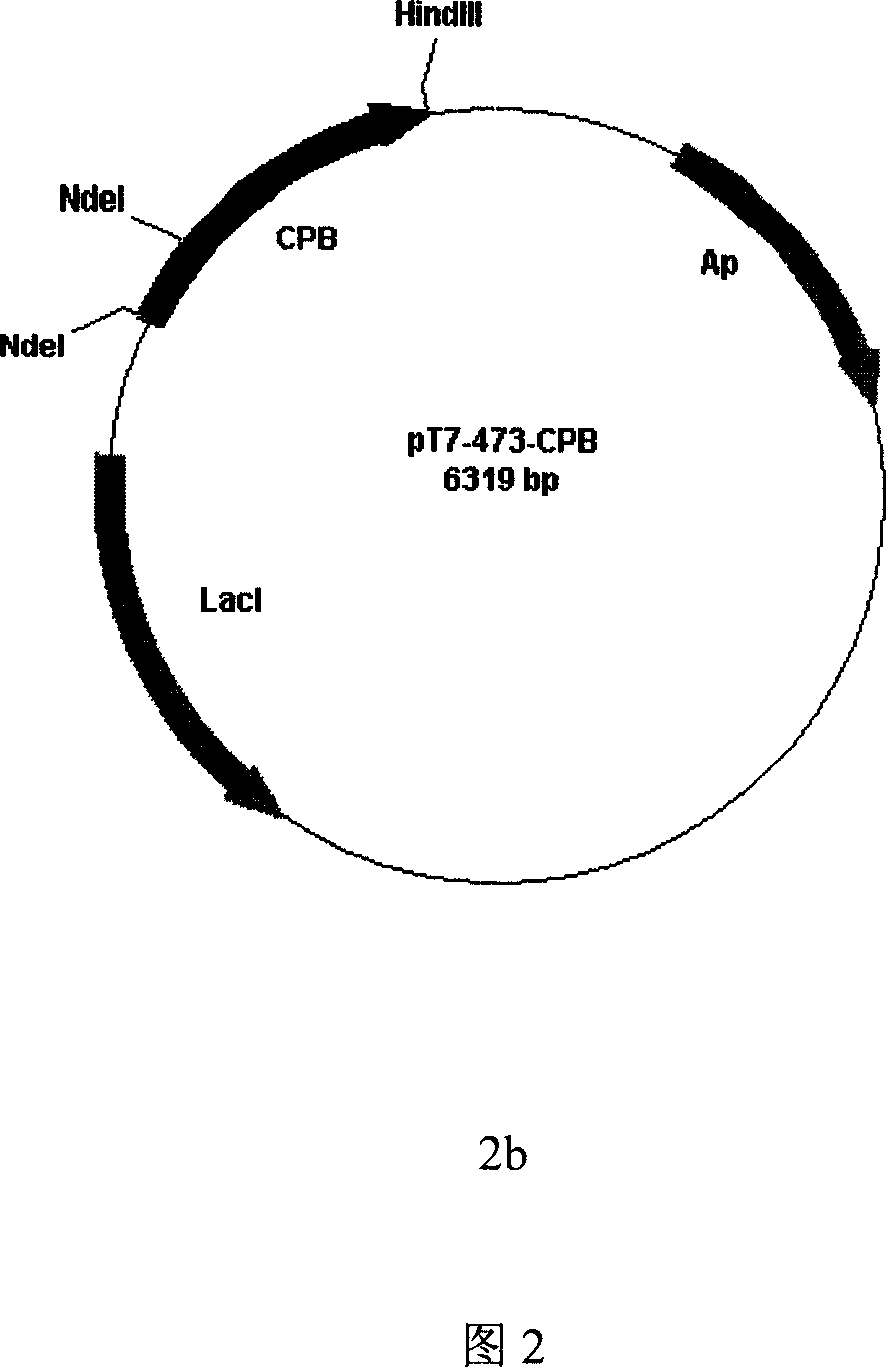
[0047] From the LB solid culture plate (LB nutrient solution, add agar powder to a concentration of 1.5 (wt%)) to pick a recombinant single colony in 5ml LB liquid nutrient solution containing 100 μg / ml ampicillin (1% (wt %) tryptone, 0.5% (wt%) yeast extract, 0.5% (wt%) NaCl, pH 7.0), 37 ° C, shake the bacteria overnight; the overnight bacteria were inoculated at 1% (v / v) in 250ml LB containing 100μg / ml ampicillin resistant In culture medium, culture at 37°C to OD 600 0.5, 0.5mmol / L IPTG induction, 1mmol / L IPTG induction, after 37 ℃ continued culture for 3 hours, harvest the bacteria, centrifuge to discard the supernatant, suspend the bacteria pellet with 20mmol / L pH8.0 TE buffer, and ultrasonically break Bacteria were centrifuged, and the precipitate was collected. It was identified by SDS-PAGE electrophoresis that the precipitate was the expression part of the inclusion body of CPB. The inclusion body was collected, suspended with 1% (v / v) Triton-X 100, fully washed, centri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com