A kind of synthetic method of gem-difluoro substituted pyrrolidone compound
A technology of pyrrolidone and synthesis method, applied in directions such as organic chemistry, to achieve the effects of wide substrate range, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Into a 35 ml sealed tube equipped with a stir bar, add 79.2 mg (0.3 mmol) of bromodifluoroacetyl-p-toluidine, 3.0 ml of acetonitrile, and 41 μL of styrene (0.36 mmol), and mix well. Then add 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 82.9 mg K 2 CO 3 (0. 6 mmol). The mouth of the tube was sealed, and the reaction was stirred at 110°C for 2 hours (the progress of the reaction was monitored by TLC). After the reaction is over, cool to room temperature, add 3ml of distilled water to the reaction mixture, extract with ethyl acetate (5ml×3), combine the organic phases, dry with anhydrous sodium sulfate, distill under reduced pressure to remove the solvent, and the residue is subjected to silica gel column chromatography (V Petroleum ether : V Ethyl acetate =30:1) 74.8 mg of white solid product 3a was isolated, with a yield of 87%. The response is as follows:
[0025]
[0026] Spectral analysis data
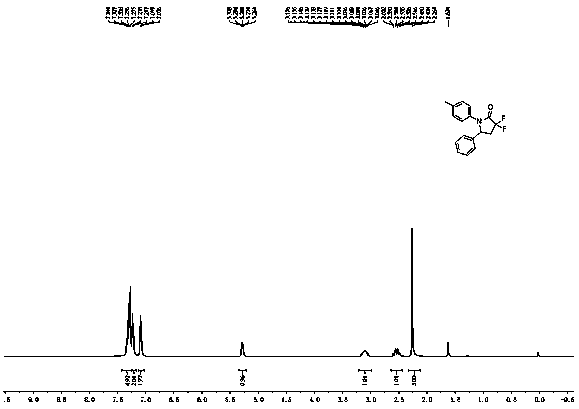
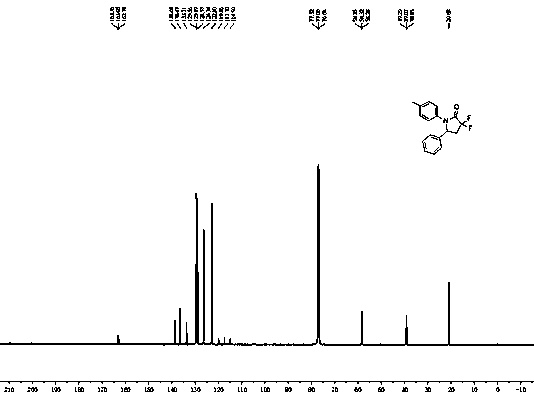
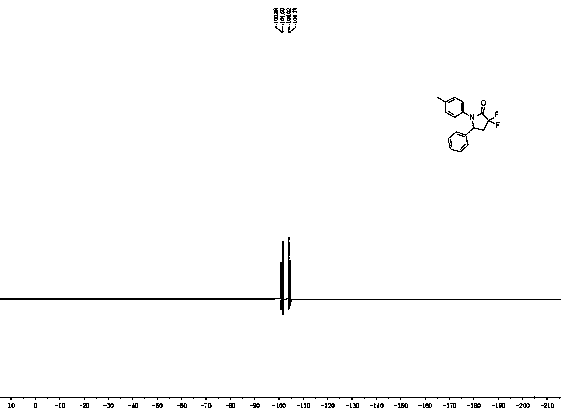
[0027] White solid. mp: 139-140 ºC. 1 H NMR (400 MHz, CDCl 3 ): δ...
Embodiment 2
[0029] Into a 35 ml sealed tube equipped with a stir bar, add 79.2 mg (0.3 mmol) of bromodifluoroacetyl-p-toluidine, 3.0 ml of acetonitrile, and 53.6 mg of 4-nitrostyrene (0.36 mmol), and mix well. Then add 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 82.9 mg K 2 CO 3 (0. 6 mmol). The mouth of the tube was sealed, and the reaction was stirred at 110°C for 2 hours (the progress of the reaction was monitored by TLC). After the reaction is complete, cool to room temperature, add 3 ml of distilled water to the reaction mixture, extract with ethyl acetate (5 ml×3), combine the organic phases, dry with anhydrous sodium sulfate, distill under reduced pressure to remove the solvent, and pass the residue through a silica gel column Analysis (V Petroleum ether : V Ethyl acetate =30:1) 91.7 mg of white solid product 3b was isolated, and the yield was 92%. The response is as follows:
[0030]
[0031] Spectral analysis data
[0032] White solid. mp: 144-146 ºC. 1 H NMR (400 MHz, CD...
Embodiment 3
[0034] Into a 35 ml sealed tube equipped with a stir bar, add 79.2 mg (0.3 mmol) of bromodifluoroacetyl-p-toluidine, 3.0 ml acetonitrile, and 62 μL eugenol methyl ether (0.36 mmol), and mix well. Then add 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 82.9 mg K 2 CO 3 (0. 6 mmol). The mouth of the tube was sealed, and the reaction was stirred at 110°C for 2 hours (the progress of the reaction was monitored by TLC). After the reaction is complete, cool to room temperature, add 3 ml of distilled water to the reaction mixture, extract with ethyl acetate (5 ml×3), combine the organic phases, dry with anhydrous sodium sulfate, distill under reduced pressure to remove the solvent, and pass the residue through a silica gel column Analysis (V Petroleum ether : V Ethyl acetate =30:1) 99.7 mg of white solid product 3c was isolated, with a yield of 60%. The response is as follows:
[0035]
[0036] Spectral analysis data
[0037] White solid. mp: 135-136 ºC. 1 H NMR (400 MHz, CDCl 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com