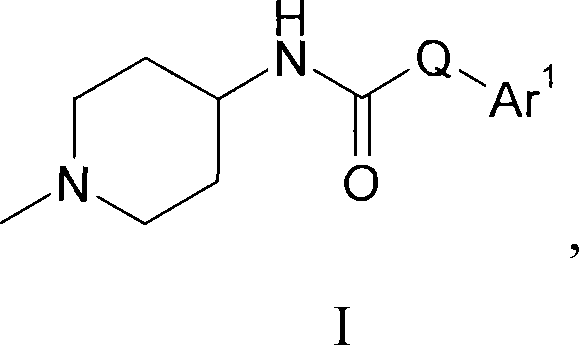
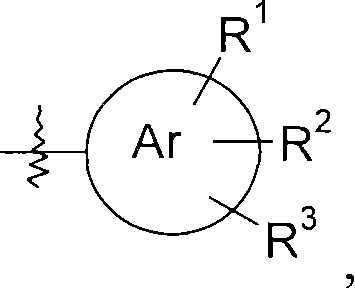
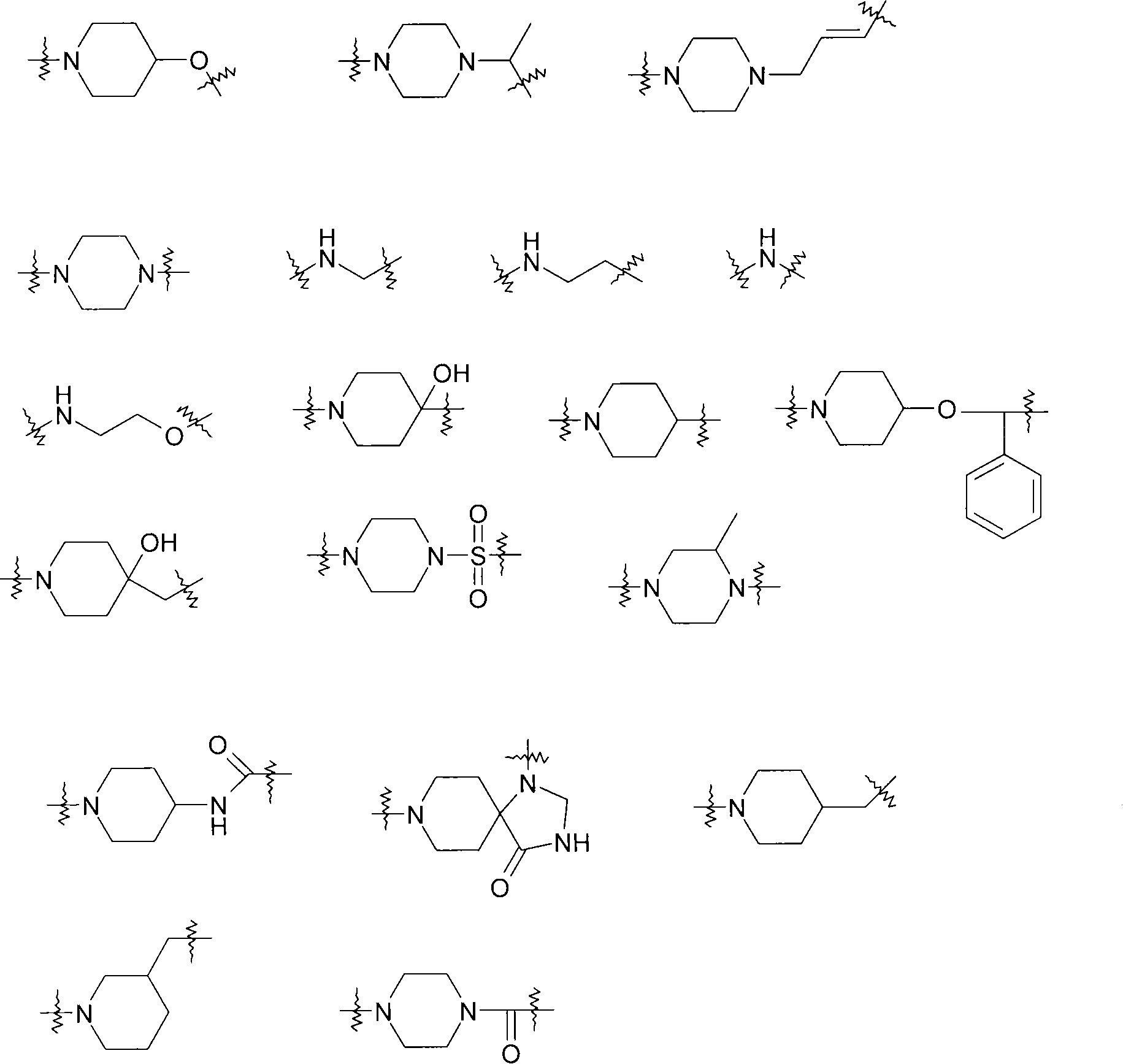Piperidine derivatives as histamine h3 receptor ligands
A technology of compounds and mixtures, applied in the field of histamine H3 receptor ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] Example 1: 1-(3,4-dichloro-benzyl)-3-(1-methyl-piperidin-4-yl)-urea
[0170] To a solution of 3,4-dichlorobenzylamine (0.195 g, 1.11 mmol) and diisopropylethylamine (0.193 mL, 1.11 mmol) in 4 mL of THF was added chloroformic acid (4-nitrophenyl ) ester (0.223 g, 1.11 mmol) in 4 mL THF pre-made solution. The reaction mixture was stirred at room temperature for 3.5 h. 4-Amino-1-methylpiperidine (0.500 g, 4.38 mmol) was added to the above solution, and the resulting solution was stirred at room temperature for 16 h. The reaction mixture was concentrated under reduced pressure, diluted with EtOAc (50 mL), and the solution was washed with saturated aqueous sodium bicarbonate (2×50 mL) and brine (50 mL). The solvent was removed under reduced pressure, and the residue was analyzed by supercritical liquid chromatography (21 mm × 150 mm diol-bonded SiO 2 (6 μm particle size), isocratic method, 25% MeOH (with 0.5% isopropylamine) / CO 2 ) to afford the title compound (0.0744 g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com