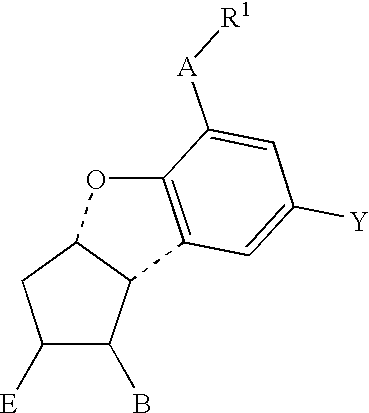
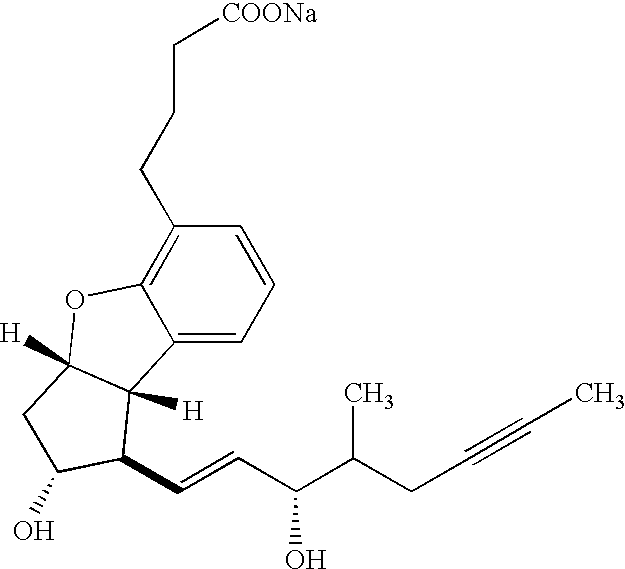
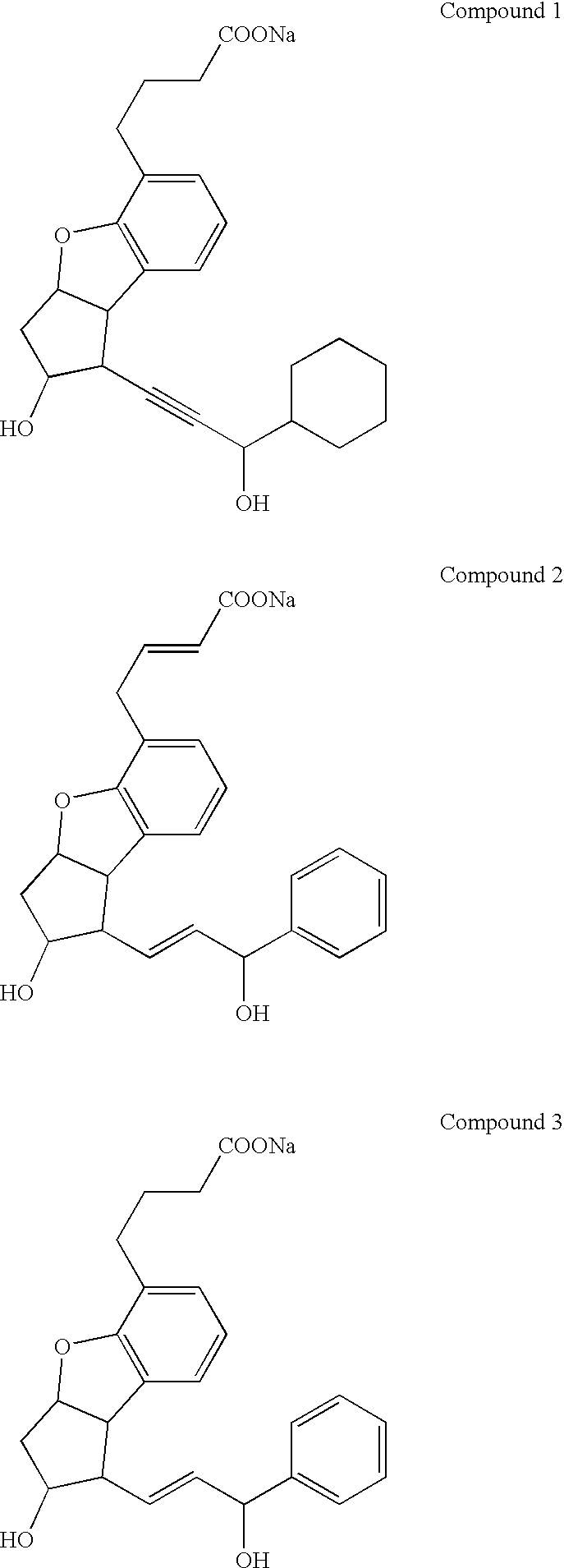
Therapeutic agent for renal failure
a technology of renal failure and therapeutic agents, which is applied in the direction of biocide, drug composition, and elcosanoid active ingredients, etc., can solve the problems of not yet recognized that such derivatives have therapeutic activities on renal failure, reduce the excretion of nitrogenous metabolic products, and fail to maintain the homeostasis of the biological environmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0096] Effect of beraprost sodium on 5 / 6 nephrectomized rat model:
[0097] The effect of beraprost sodium on a 5 / 6 nephrectomized rat model, which has been widely used as a model animal for renal failure, was examined. Two-thirds of the left kidney was removed from each of 4-week-old male Wistar rats (Charles River Japan Inc.) with a razor, and all of the right kidney was then removed therefrom one week after. Three weeks after the initial surgery, blood was collected from each rat through the tail vein and serum creatinine and BUN levels were determined from the blood. At this point of time, the urine was also collected for 24 hours to determine the mass of proteins in the urine. The rats were allocated to one of the treatment groups by the stratifying continuous randomization method based on the mass of proteins in the urine and the body weight (n=8 per group). There was observed little difference in the initial values of blood creatinine and BUN values among the rats. Three weeks a...
example 2
[0100] Renal failure rat models of which the primary disease was glomerulonephritis were used to examine the effect of different 4,8-inter-m-phenylene prostaglandin I.sub.2 derivatives including beraprost sodium on the models. Eight-week-old male WKY rats (Charles River Japan Inc.) were administered intravenously with rabbit anti-rat glomerular basement membrane antiserum to induce glomerulonephritis. Two weeks after the induction, blood was collected from each rat through the tail vein to determine blood creatinine and BUN levels. The blood creatinine and BUN levels in the glomerulonephritis-induced rats were remarkably higher than those in the non-induced rats, which indicated that the conditions of the rats progressed into renal failure. Four types of 4,8-inter-m-phenylene prostaglandin I.sub.2 derivatives in total, including beraprost sodium, were individually administered subcutaneously to the rats from the back continuously with an osmotic pump (ALZET) for one week, beginning ...
example 3
[0103] Glomerulonephritis rat models were used to examine the effect of beraprost sodium on the rat models both in a stage where renal failure had not been found (i.e., the inflammatory stage) and a stage where BUN level was increased and the conditions were progressed into renal failure (i.e., the renal failure stage). Eight-week-old male WKY rats (Charles River Japan Inc.) were administered intravenously with rabbit anti-rat glomerular basement membrane antiserum to induce glomerulonephritis. Each of beraprost sodium, captopril (SIGMA) and prednisolone (Shionogi & Co., Ltd.) was orally administered to the rats everyday either for one week from day 1 through day 7 after induction of glomerulonephritis (i.e., during the inflammatory stage) or for two weeks beginning two weeks after the induction through four weeks after the induction (i.e., during the renal failure stage). The frequency of the administration was twice a day for beraprost sodium and captopril and once a day for predn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com