Composition for preventing and/or treating renal injury and renal failure
A composition and a technique for renal failure, applied in the field of medicine, can solve problems such as the treatment and prevention of renal function diseases that have not been seen in its application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of lyophilized formulations
[0037] Active ingredient: Utilize conventional gene recombination technology to produce recombinant MG53 protein, the method is as follows:
[0038] Firstly, total RNA (total RNA) was extracted from human skeletal muscle or cardiac tissue, and then a part of PCR amplification was performed with specific primers of MG53, and then the PCR product was directly purified and sequenced. After the sequence was confirmed to be correct, the fragment was combined with eukaryotic In the expression vector pcDNA4 / V5-His A, the vector was transformed into the 293T cell line with a transfection reagent, and then the stable expression cell line (293T-MG53) was screened using the Zeocin selection marker. Expand and cultivate the cell line, and then undergo separation and purification steps such as affinity chromatography and ion exchange chromatography to obtain MG53 protein with a purity of more than 95%. The N-terminal sequence of the protein ...
Embodiment 2
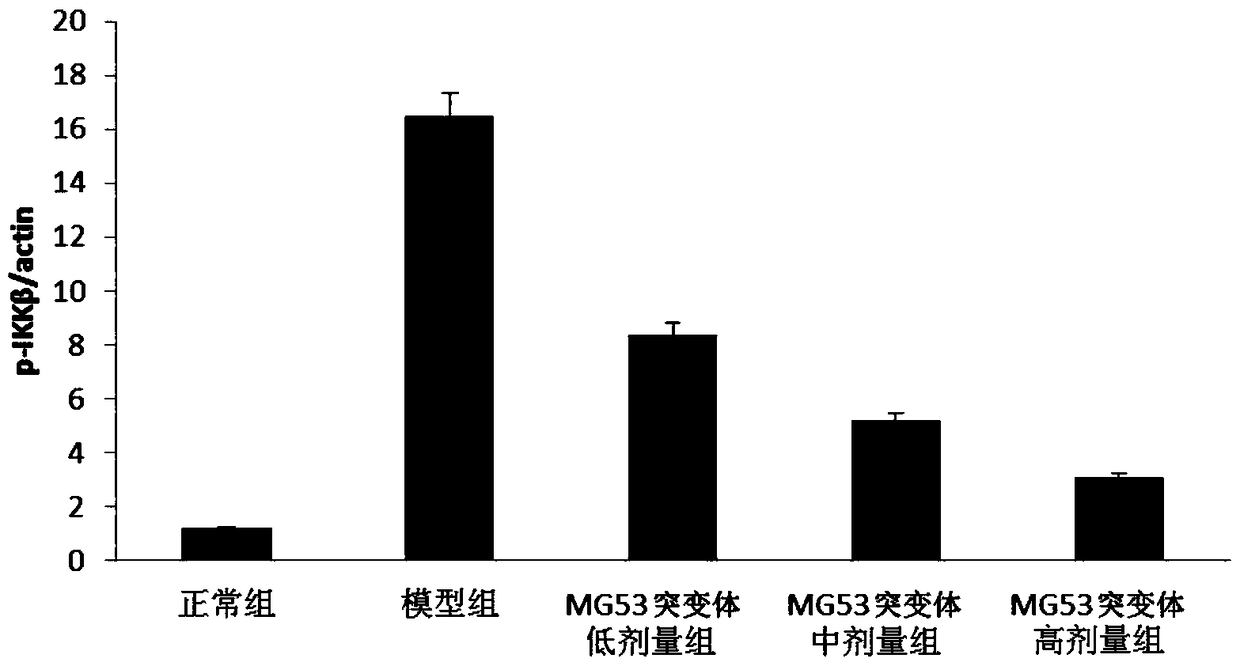
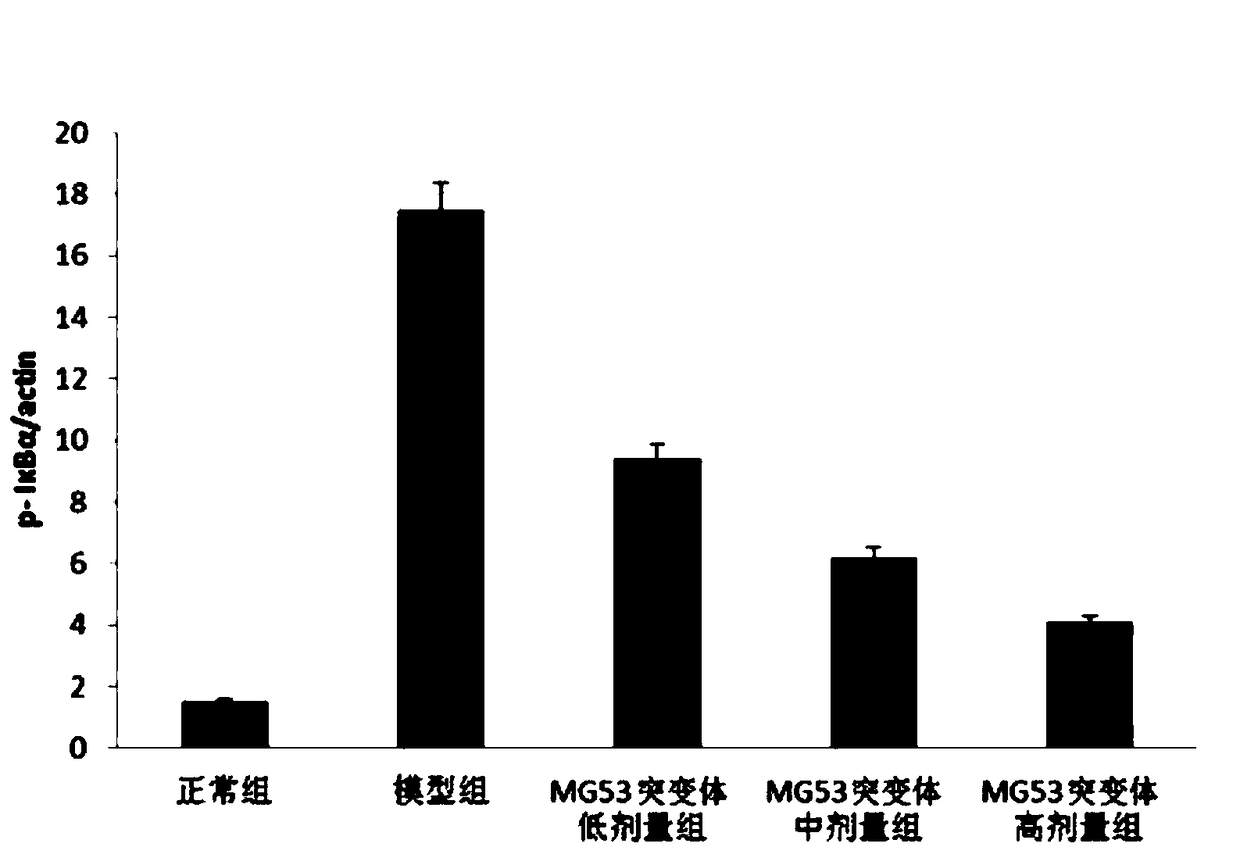
[0042] Effects of MG3 protein mutants on mouse models of acute kidney injury
[0043] Objective: To study the effect of MG53 protein mutants using a mouse model of acute kidney injury
[0044] Materials and methods: BALB / c mice, male, weighing 18-22g, were randomly divided into normal control group, model control group, low-dose group (0.5mg / kg), middle-dose group (2mg / kg), high-dose group Dose group (5mg / kg), 15 rats in each group.
[0045] The experimental group was intragastrically administered a corresponding amount of the composition, and the normal control group and the model control group were intravenously injected with corresponding volumes of normal saline. Once a day, continuous administration for 7 days. After the last administration, except the normal control group, the other groups were intraperitoneally injected endotoxin at 50 mg / kg to establish the endotoxin-acute kidney injury model, and the normal control group was injected with the same dose of normal sal...
Embodiment 3
[0079] Effects of MG3 protein mutants on nephrectomy-induced chronic renal failure in rats
[0080] Objective: To study the effect of MG53 protein mutants on chronic renal failure in rats
[0081] Materials and methods: 120 rats, weighing 150-200g, fed the animals for 1 week, divided the animals into 6 groups, 20 rats in each group, namely the normal group, the model group, and the glutathione intravenous injection (150mg / kg) group , MG53 mutant low-dose group, MG53 mutant medium-dose group, MG53 mutant high-dose group, in addition to the normal group, rats were made into chronic renal failure models by 5 / 6 nephrectomy,
[0082] 2 / 3 of the left kidney was resected in the first operation, and the right kidney was resected in the second operation one week later. Drugs were started 5 weeks after the second operation. The MG53 mutant was administered by tail vein injection. Blood was collected from the eye sockets of the rats at 1 week and 8 weeks respectively, and serum creatini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com