Chimeric peptides for the regulation of GTPases
a technology of gtpase and chimeric peptides, which is applied in the direction of peptide sources, antibody medical ingredients, drug compositions, etc., can solve the problems of preventing effective cycling between and negatively affecting the activity of rho protein, and achieve the effects of modulating gtpase activity, inhibiting gtpase activity, and reducing gtpase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Distinct Roles of RhoA, RhoB and RhoC in Cell Transformation and Migration
[0274] Among the three closely related Rho proteins, RhoA, RhoB and RhoC, RhoA is the best characterized one and has been shown to regulate actin stress fiber and focal complex formation, to promote cell growth and to transform NIH 3T3 fibroblasts. By contrast, the function of RhoB or RhoC in fibroblasts has not been examined in detail and has not been directly compared with that of RhoA. To make comparisons of the cellular roles of RhoA, RhoB and RhoC, two sets of activating mutants were generated for each of the Rho proteins: the fast-cycling Rho-F30L and the GTPase-defective Rho-Q63L. Both types of mutants result in the net enhancement of the active Rho-GTP species in cells but involved distinct mechanisms: Rho-F30L proteins contained significantly increased intrinsic GDP / GTP exchange activity and remained responsive to RhoGAP stimulation to cycle between the GDP- and GTP-bound states, while the Rho-Q63L m...
example 2
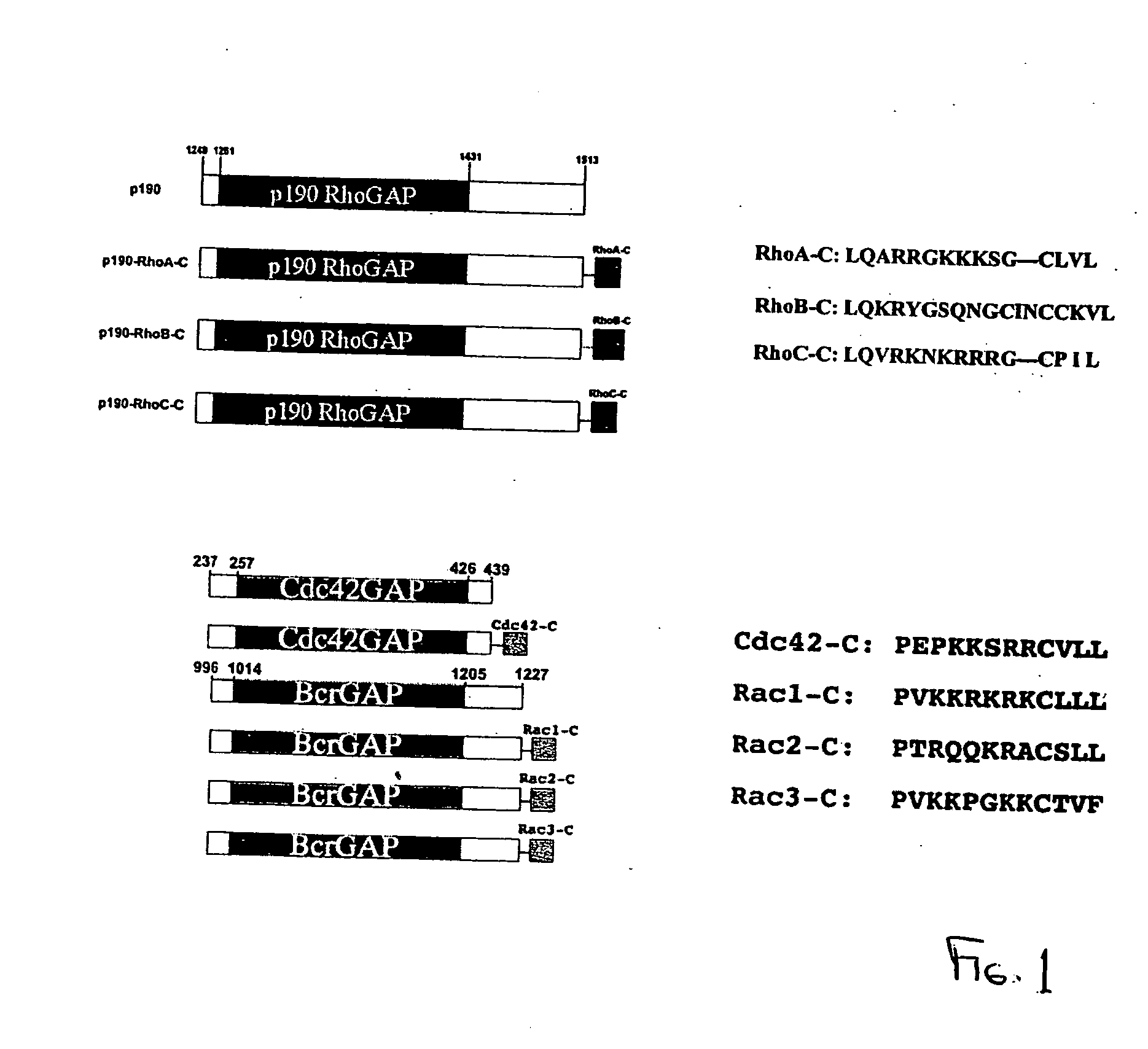
Targeting Individual Rho GTPase Activities by p190-Rho Chimeras
[0283] The commonly used biochemical tools to implicate the involvement of a Rho protein in a particular signaling pathway include the dominant negative mutant of the Rho protein and certain bacterial toxins that can modify the Rho protein function. These reagents are limited by their non-specific nature in interfering with Rho GTPase functions (Geig, et al. 1999, Nat. Cell. Biol. 1, E25-E27, herein incorporated by reference in its entirety) and can have limited therapeutic value in targeting specific Rho proteins. In order to specifically inhibit individual Rho protein function, we hypothesize that the negative regulatory role of RhoGAPs, the RhoGAP domain in particular, could be exploited to downregulate Rho protein activity if it is directed to where the active Rho GTPase substrates reside in cells. The RhoGAP domain of p190 has previously been demonstrated as a catalyst to specifically stimulate GTP-hydrolysis of Rh...
example 3
Comparison of GAP Activities of Fusion Proteins In Vitro
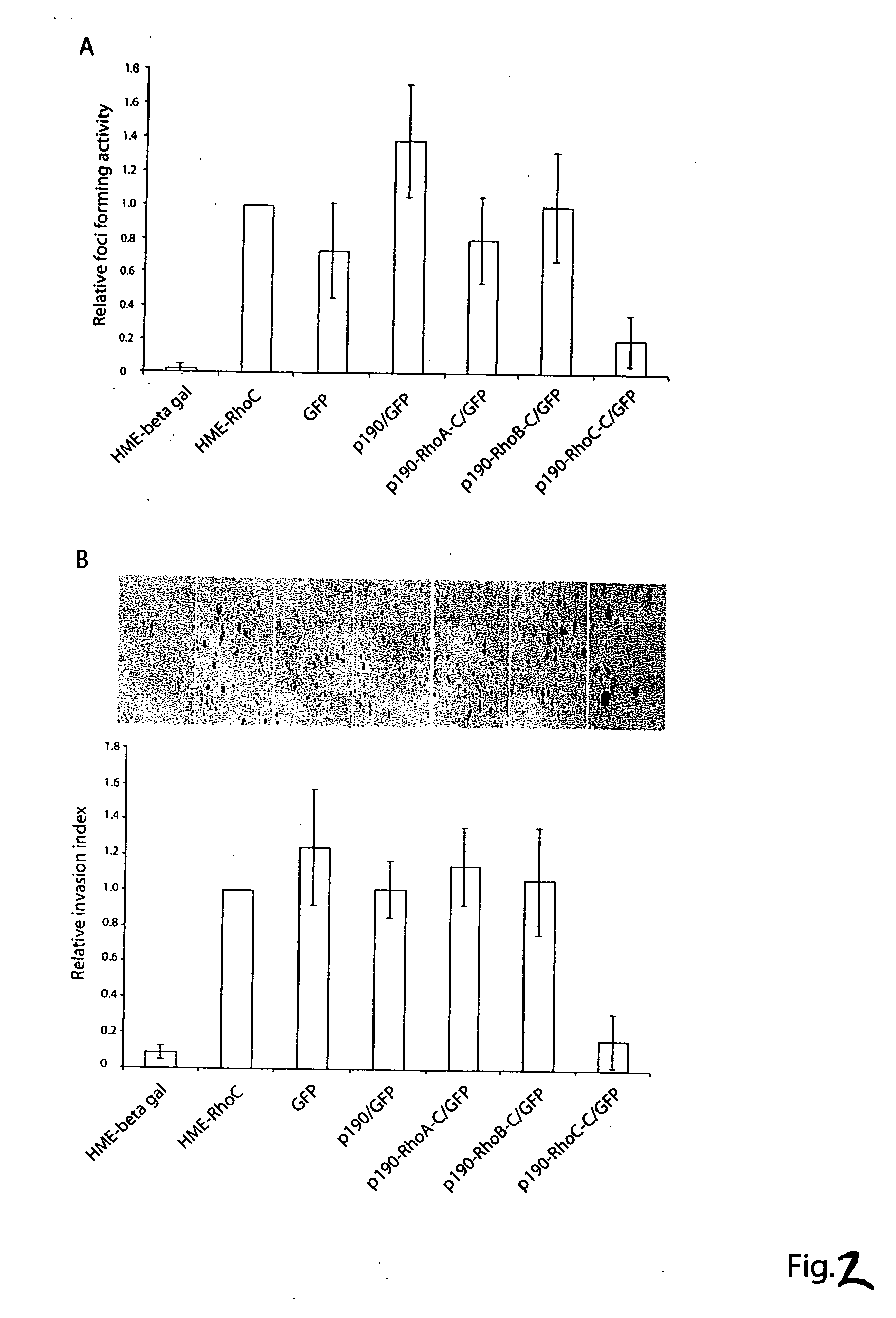
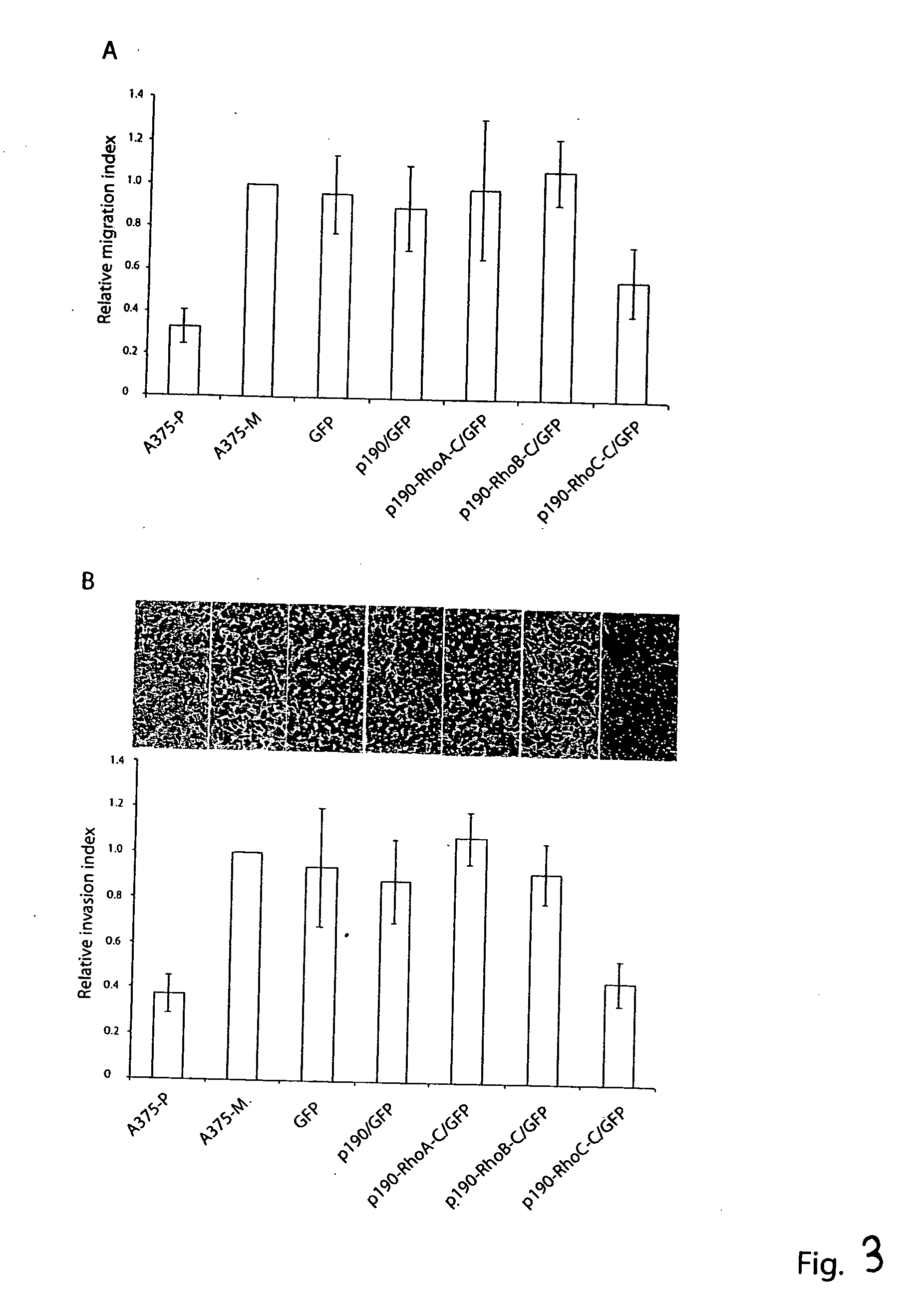
[0285] To ensure that the C-terminal sequences of various Rho proteins fused to the GAP domain of p190 do not interfere with the GAP function of p190, we expressed the GST-tagged p190 and p190-Rho chimeras in Cos-7 cells, purified them by glutathione-agarose affinity beads and compared their GAP activities in vitro. Under similar doses, p190-RhoA-C, p190-RhoB-C and p190-RhoC-C displayed comparable GAP activities as p190 GAP domain alone on the RhoA substrate to stimulate [γ32P]GTP-hydrolysis in a time dependent manner, indicating that all four p190 and p190-Rho chimera proteins are functional. To evaluate the efficacy and specificity of the chimeric constructs in cells, we employed the above described fast-cycling mutant Rho-F30L and GTPase-defective mutant Rho-Q63L expressing cells as testing systems, taking advantage of the facts that the F30L mutant form of the Rho GTPases is fully responsive to GAP stimulation while the GT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com