Hydrophobically-modified protein compositions and methods
a technology of hydrophobic modification and protein composition, which is applied in the field of hydrophobic modification protein composition and method, can solve the problems of low affinity of a protein for its receptor, inactive coupled proteins, and low specificity of methods, so as to increase the biological activity and/or stability of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Human Sonic Hedgehog is Lipid-Modified when Expressed in Insect Cells
[0208] A. Expression of Human Sonic Hedgehog
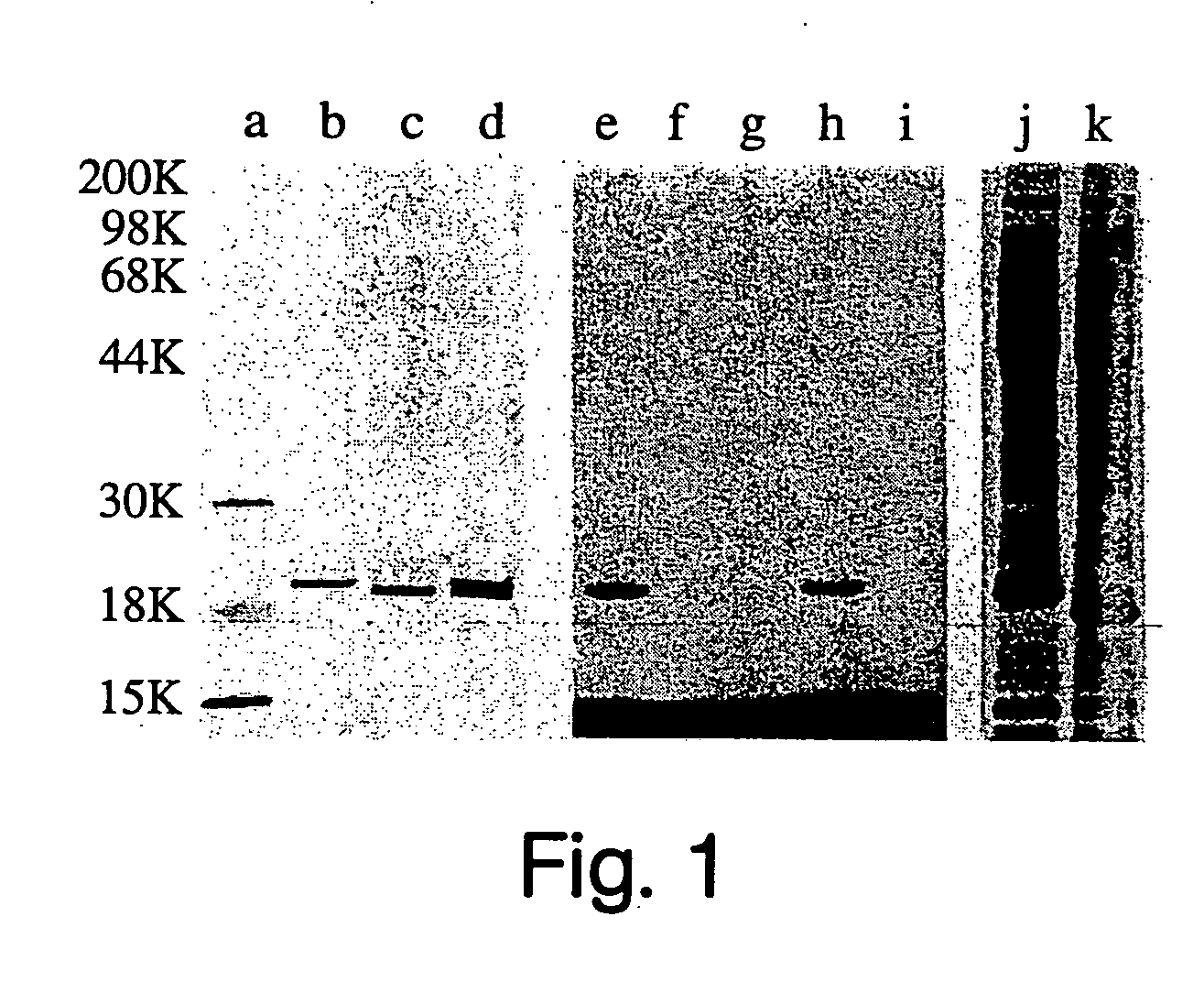
[0209] The cDNA for fill-length human Sonic hedgehog (Shh) was provided as a 1.6 kb EcoRI fragment subcloned into pBluescript SK+ (20) (a gift of David Bumcrot from Ontogeny, Inc., Cambridge Mass.). The 5′ and 3′ NotI sites immediately flanking the Shh open reading frame were added by unique site elimination mutagenesis using a Pharmacia kit following the manufacturer's recommended protocol. The 1.4 kb NotI fragment carrying the full-length Shh cDNA was then subcloned into the insect expression vector, pFastBac (Life Technologies, Inc.). Recombinant baculovirus was generated using the procedures supplied by Life Technologies, Inc. The resulting virus was used to create a high titer virus stock. Methods used for production and purification of Shh are described below. The presence of membrane-associated Shh was examined by FACS and by Western blot analysis. Peak expressio...
example 2
Human Sonic Hedgehog can be Modified with Palmitic Acid in a Cell-Free System
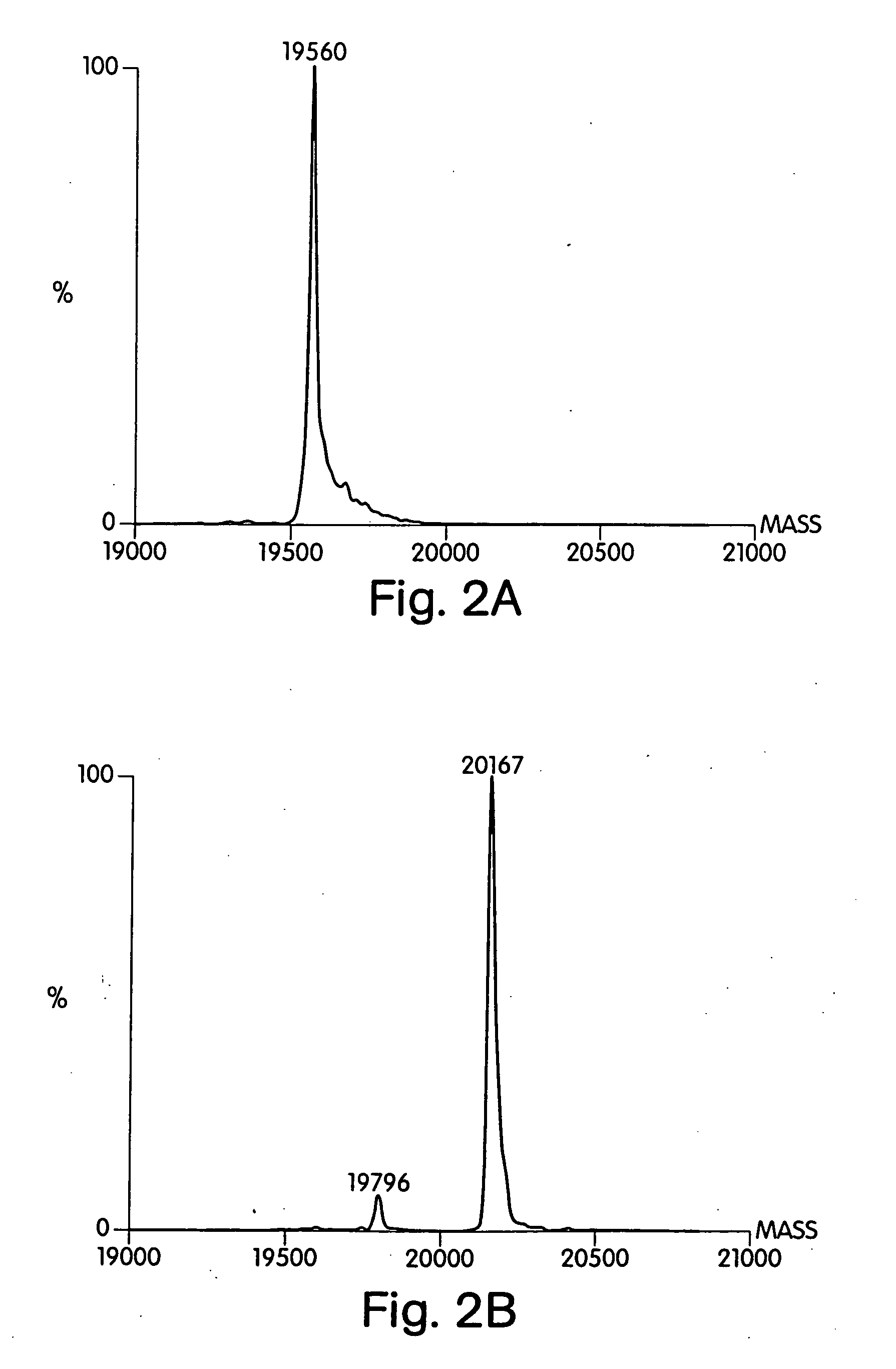
[0224] Soluble Shh was labeled with 3H-palmitic acid in a cell-free system using a modified version of a published procedure (24). A crude microsomal fraction from rat liver was prepared by subjecting a liver homogenate to sequential centrifugation at 3000×g for 10 min, 9000×g for 20 min, and 100,000×g for 30 min. The 100,000×g pellet was suspended in 10 mM HEPES pH 7.4, 10% sucrose and again centrifuged at 100,000×g for 20 min. The final pellet (derived from 10 g of liver) was suspended in 3 mL of 20 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 10 μg / mL leupeptin, 0.15% Triton X-100, aliquoted, and stored at −70° C. Reactions containing 3 μg Shh, 1 μL of rat microsomes, 50 ng / mL Coenzyme A (Sigma), 0.3 mM ATP, 20 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 10 μg / mL leupeptin, and 0.5 μCi-[9,10-3H]-palmitic acid (50 Ci / mmol; New England Nuclear) were performed at room temperature for 1 h. Reactions were sto...
example 3
Demonstration of Increased Potency of Naturally Ocurring Fatty-Acylated Human Sonic Hedgehog in a Cell-Based (C3H10T1 / 2) Assay
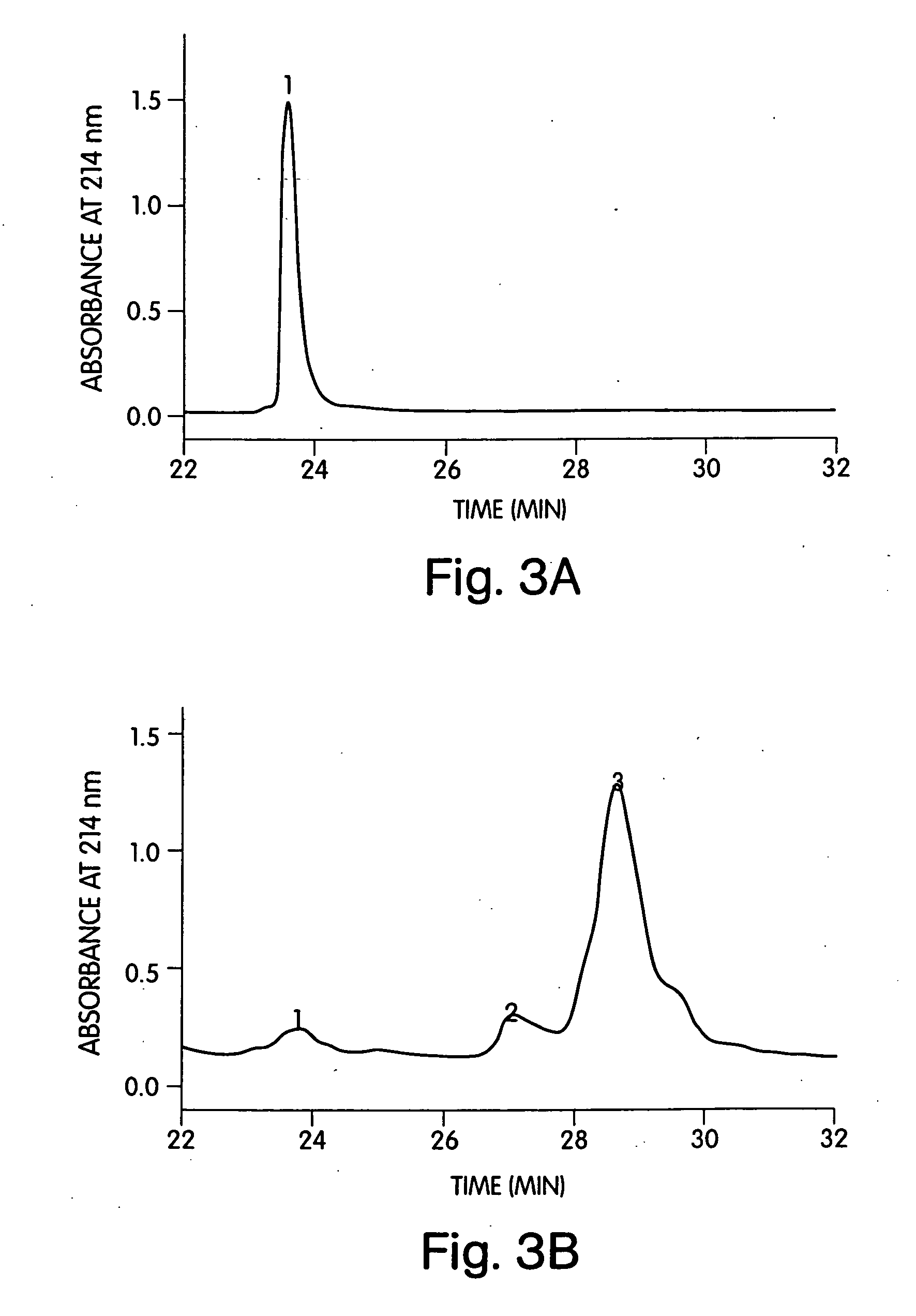
[0228] Shh was tested for function in a cell-based assay measuring alkaline phosphatase induction in C3H10T1 / 2 cells (25) with a 5 day readout. The assay was preformed in a 96-well format. Samples were run in duplicate. For tethered Shh (100 μg / mL), the samples were first diluted 200-fold with normal growth medium then subjected to serial 2-fold dilutions down the plates. Wells were normalized for potential effects of the added octylglucoside by including 0.005% octylglucoside in the culture medium. Blocking studies using the neutralizing murine mAb 5E1 (26) were performed by mixing Shh with serial dilutions of the antibody for 30 min at ambient temperature in culture medium prior to adding the test samples to the plates.
[0229] In this assay, soluble human Shh produces a dose-dependent response with an IC50 of 1 μg / mL and a maximal signal at 3 μg / mL (FIG. 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal diameter×250 | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com