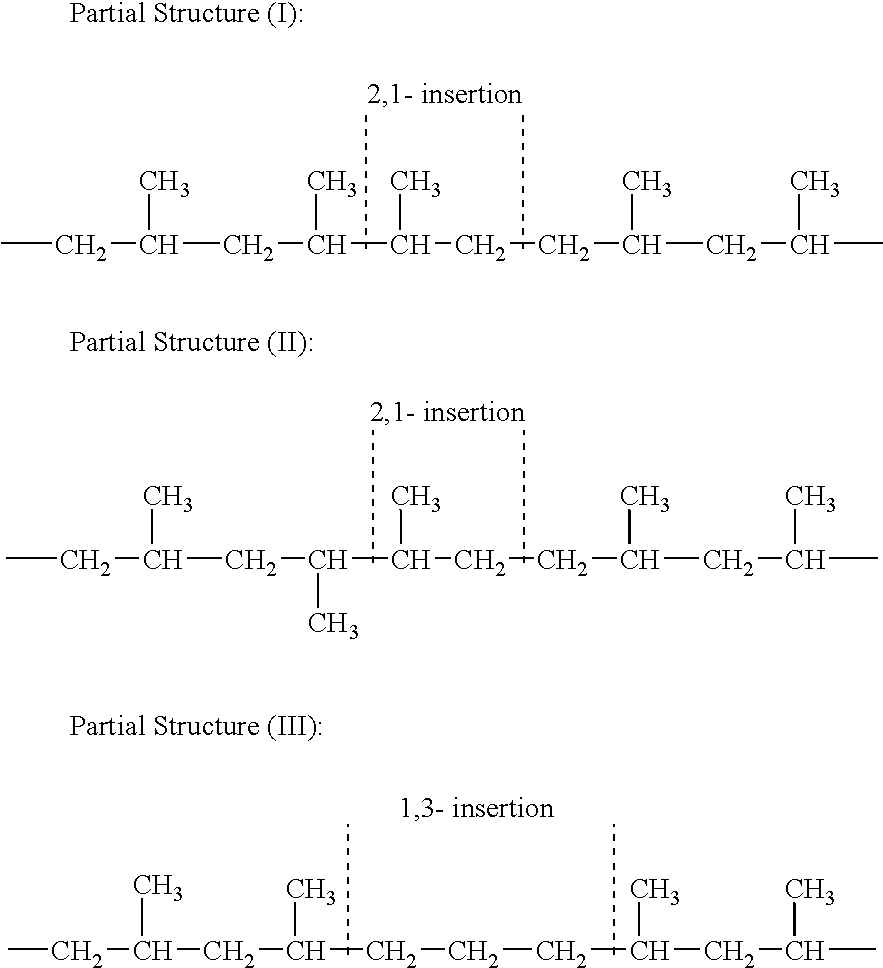
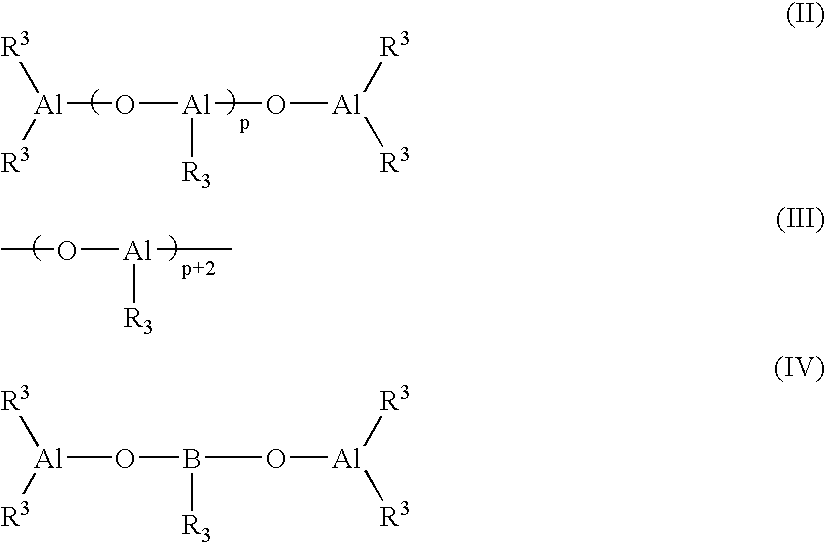
Soft propylene-based resin composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(1) Production of dichloro{1,1′-dimethylsilylene[2-methyl-1-indenyl]-[2-methyl-4-phenyl-4H-azulenyl]}hafnium:
[0407] The following process was all carried out in a dry nitrogen atmosphere, according to ordinary Schlenk techniques. Toluene, tetrahydrofuran, and diethyl ether for solvent were dried over benzophenone ketyl with sodium metal before use. N-hexane used herein was dehydrated n-hexane by Kanto Chemical.
[0408] A n-hexane solution of n-butyllithium (50.0 ml, 79.5 mmols, 1.59 N) was dropwise added to a suspension of 2-methylindene (9.8 g, 75.3 mmols) and n-hexane (100 ml) through a syringe at −70° C., over 10 minutes. With stirring, this was gradually warmed up to room temperature, over 2 hours. This was further stirred for 20 hours at room temperature, and the solid component was removed by filtration from the resulting pale yellow suspension via a cannula. The thus-filtered solid was washed twice with 30 ml of n-hexane, and then dried under reduced pressure to a constant w...
example 2
(1) Production of dichloro{1,1′-dimethylsilylene[2-methyl-1-indenyl]-[2-methyl-4-phenyl-4H-azulenyl]}zirconium:
[0427] A n-hexane solution of n-butyllithium (9.4 ml, 14.9 mmols, 1.59 N) was added to a n-hexane solution (120 ml) of dimethyl(2-methyl-1-indenyl)(2-methyl-4-phenyl-4H-1-azulenyl)silane (2.9 g, 7.1 mmols) obtained in Example 1(1), at 0° C. through a syringe, over 5 minutes. Next, this was stirred at 0° C. for 1.5 hours, and then at room temperature for 17.5 hours. The resulting suspension was filtered through a cannula, and the solid thus obtained was washed twice with 30 ml of n-hexane. The solid was dried under reduced pressure to give an yellow brown powder, dimethyl(2-methyl-1-indenyl)(2-methyl-4-phenyl-4H-1-azulenyl)silane dilithium salt (3.0 g).
[0428] The resulting dilithium salt (0.42 g, 1.0 mmol) and zirconium tetrachloride (0.23 g, 1.0 mmol) were metered in an inert gas atmosphere in a glove box, and put into a 100-ml round-bottom flask (equipped with a magneti...
example 3
(1) Production of dichloro{1,1′-dimethylsilylene[2-methylbenzo[e]indenyl]-[2-methyl-4-phenyl-4H-azulenyl]}hafnium:
[0449] 2-Methylbenzo[e]indene (1.87 g, 10.4 mols) was dissolved in dehydrated n-hexane (38 ml), and n-butyllithium (1.59 M, 6.6 ml, 10.5 mmols) was added to the resulting solution at 0° C. through a syringe. The solution was at first transparent and gradually became cloudy, and formed a precipitate. The white suspension was stirred at room temperature for 24 hours and filtered through a cannula. The resulting white solid was washed with dry n-hexane (10 ml×2), and dried under reduced pressure to obtain a spongy white solid (1.84 g, yield 95%).
[0450] The thus-obtained white solid (2-methylbenzo[e]indenyllithium, 0.94 g, 5.1 mmols) was dissolved in dry tetrahydrofuran / diethyl ether (40 ml, 1 / 1 v / v). On the other hand, dimethyldichlorosilane (2.60 g, 20.2 mmols) was dissolved in dry tetrahydrofuran / diethyl ether (20 ml, 1 / 1 v / v), and the resulting solution was dropwise a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap