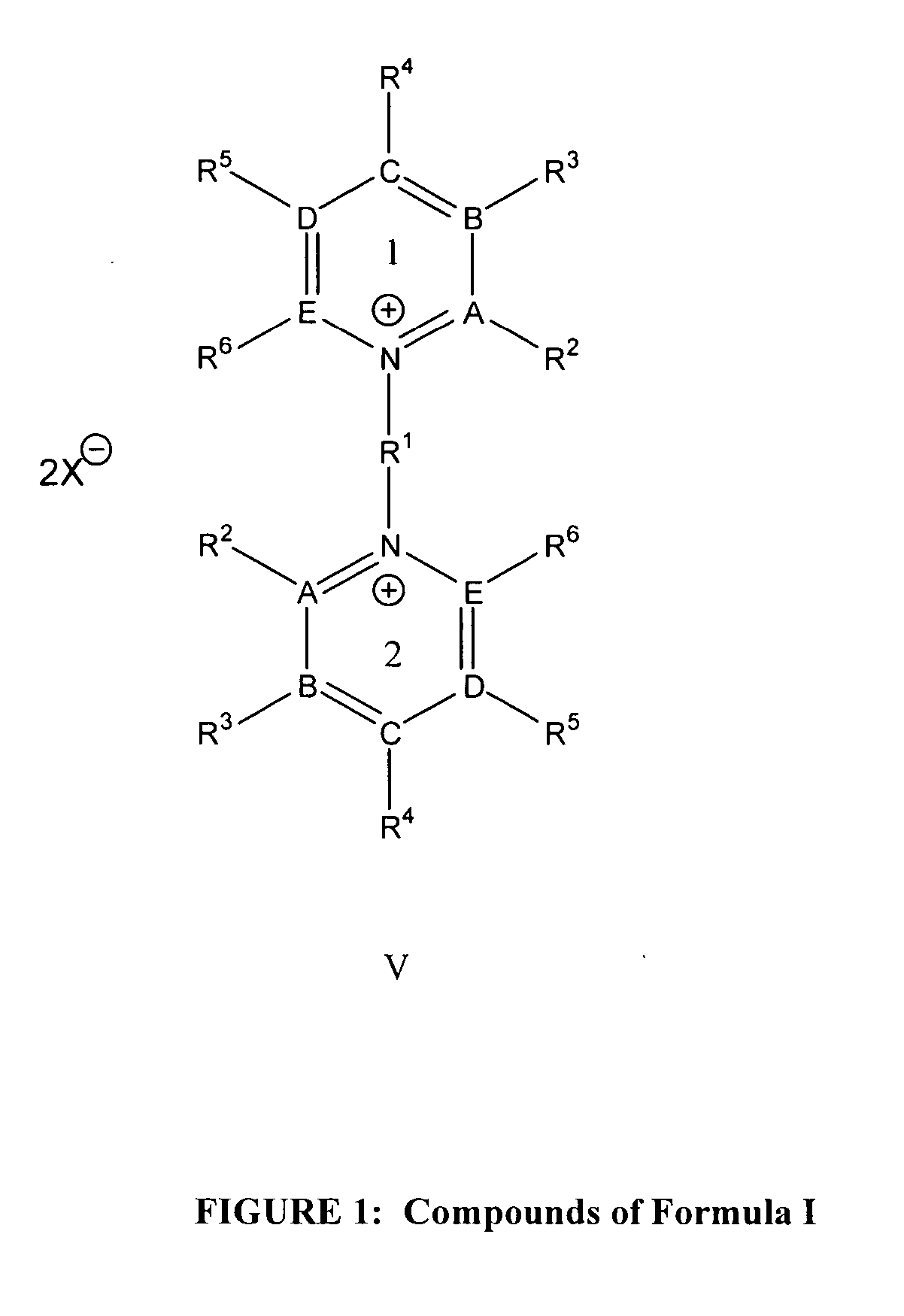
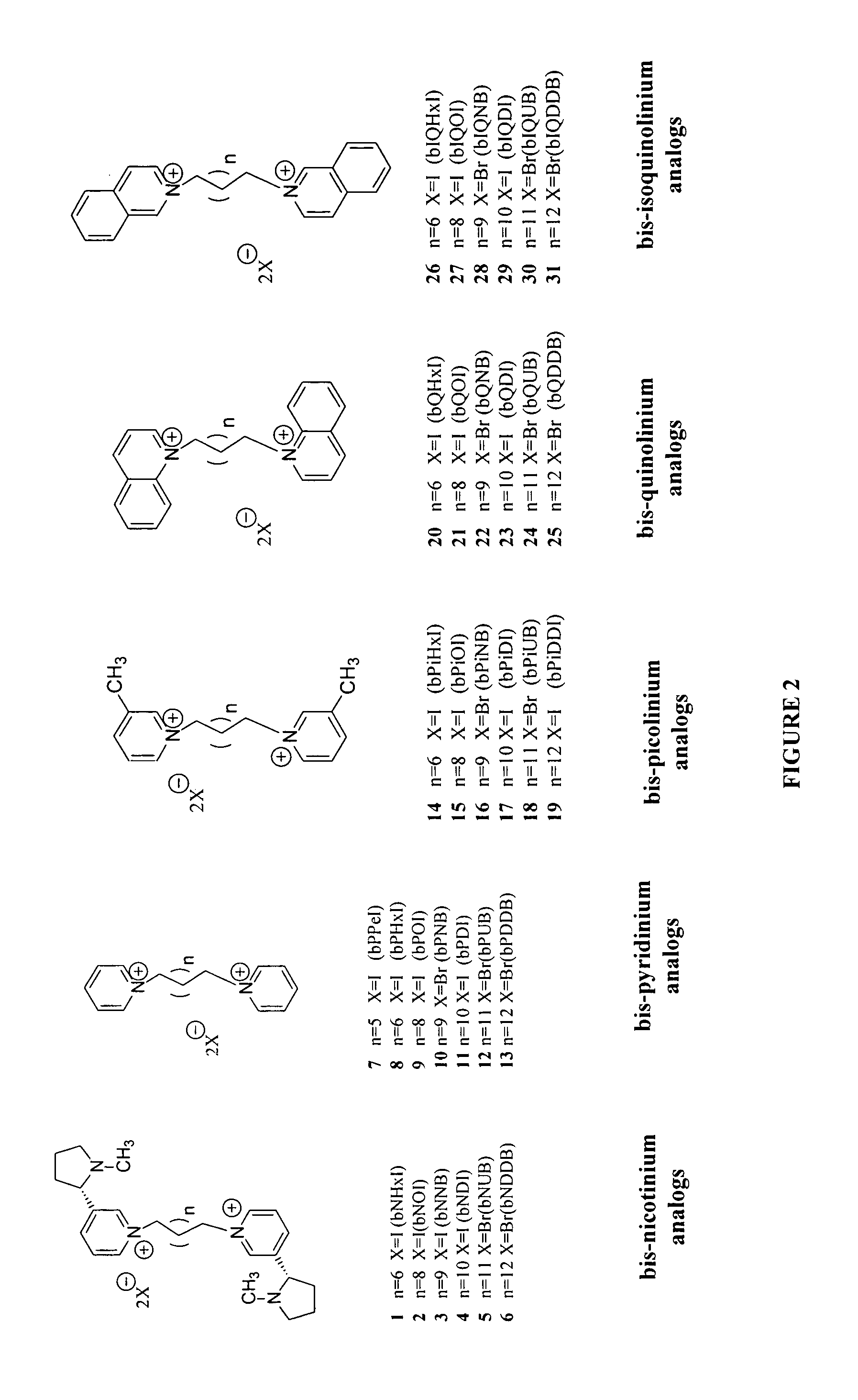
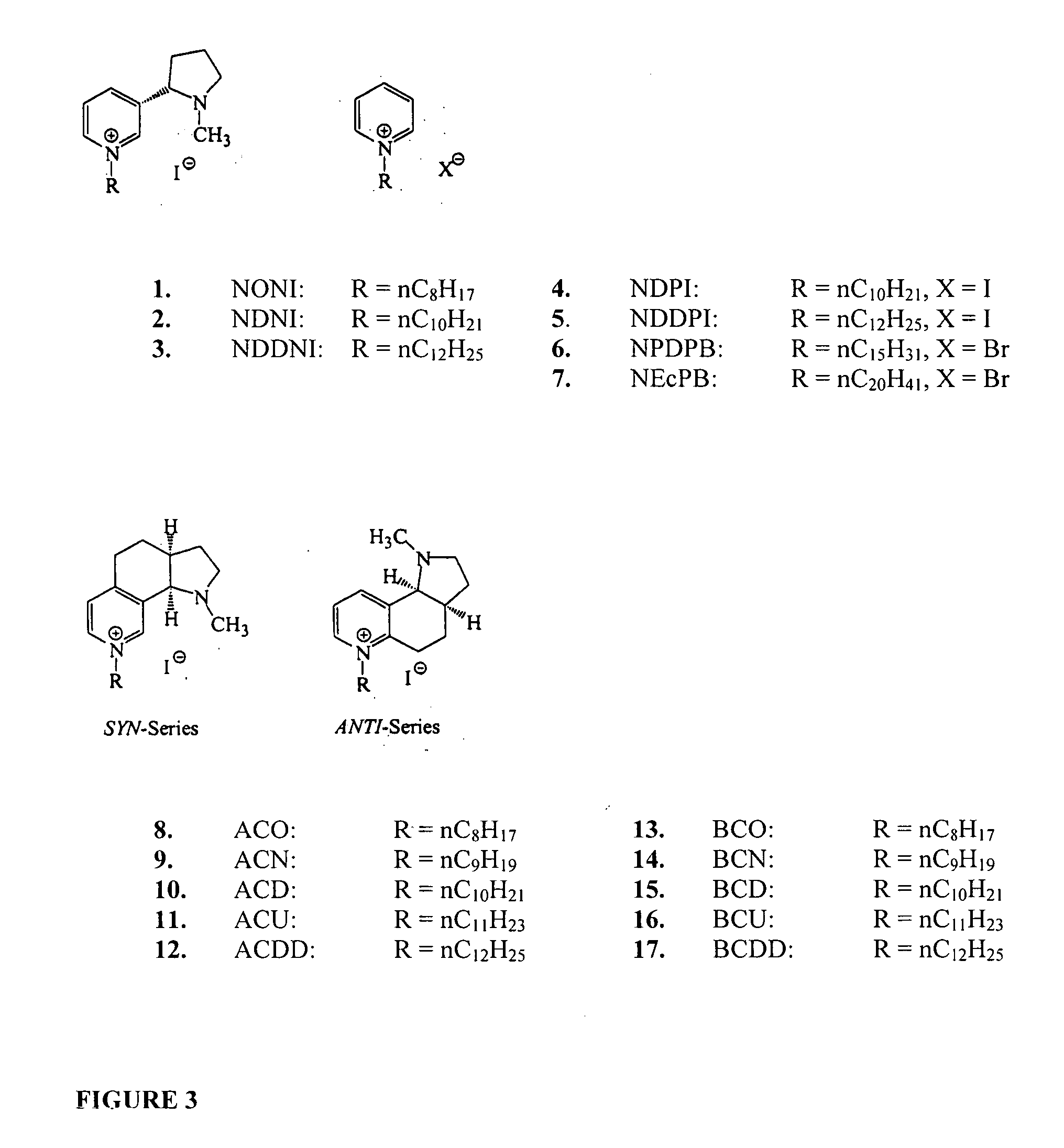
Bis-pyridino containing compounds for the use in the treatment of CNS pathologies
a technology of cns pathologies and bispyridino, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems that quaternary ammonium compounds cannot easily access the brain, and relatively few studies have focused on the therapeutic development of nachr antagonists, etc., to achieve the effect of inhibiting dopamine releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0084] N,N′-Pentane-1,5-diyl-bis-pyridinium Diiodide (bPPeI).
[0085] 1,5-Diiodopentane (mmol) was added to a solution (30 mL) of dry pyridine, and the solution heated for 24 hours at 65° C. The resulting precipitate was filtered, and the product washed five times with dry diethyl ether. The resulting yellow solid was isolated in a 90% yield. 1H NMR (300 MHz, DMSO-D6) δ 9.14 (2H, d, C2&C6-H), 8.62 (1H, t, C4-H), 8.19 (2H, t, C3&C5-H), 4.62 (2H, t, C′1-CH2), 1.92 (2H, m, C′2-CH2), 1.25 (1H, m, C′3-CH2).
example 2
[0086] N,N′-Hexane-1,6-diyl-bis-pyridinium Diiodide (bPHxI).
[0087] 1,6-Diiodohexane (mmol) was added to a solution (30 mL) of dry pyridine, and the solution heated for 24 hours at 65° C. The resulting precipitate was filtered, and the product washed five times with dry diethyl ether. The resulting yellow solid was isolated. 1H NMR (300 MHz, DMSO-D6) δ 9.11 (2H, d, C2&C6-H), 8.63 (1H, t, C4-H), 8.18 (2H, t, C3&C5-H), 4.59 (2H, t, C′1-CH2), 1.89 (2H, m, C′2-CH2), 1.28 (2H, m, C′3-CH2).
example 3
[0088] N,N′-Octane-1,8-diyl-bis-pyridinium Diiodide (bPOI).
[0089] 1,8-Diiodooctane (mmol) was added to a solution (30 mL) of dry pyridine, and the solution heated for 24 hours at 65° C. The resulting precipitate was filtered, and the product washed five times with dry diethyl ether. The resulting yellow solid was isolated in a 93% yield. 1H NMR (300 MHz, DMSO-D6) δ 9.11 (2H, d, C2&C6-H), 8.63 (1H, t, C4-H), 8.18 (2H, t, C3&C5-H), 4.59 (2H, t, C′1-CH2), 1.89 (2H, m, C′2-CH2), 1.28 (4H, m, C′3&4-CH2); 13C NMR (75 MHz, DMSO-D6) δ 145.3, 144.5, 127.9, 60.5,30.6, 28.1, 25.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com