Methods and products related to treatment and prevention of hepatitis C virus infection
a technology products, applied in the field of methods and products related to the treatment and prevention of hepatitis c virus infection, can solve the problems of poor results (about 40% sustained response) for genotype 1 hcv, difficult compilation of epidemiological statistics, and inconsistently high sustained response rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
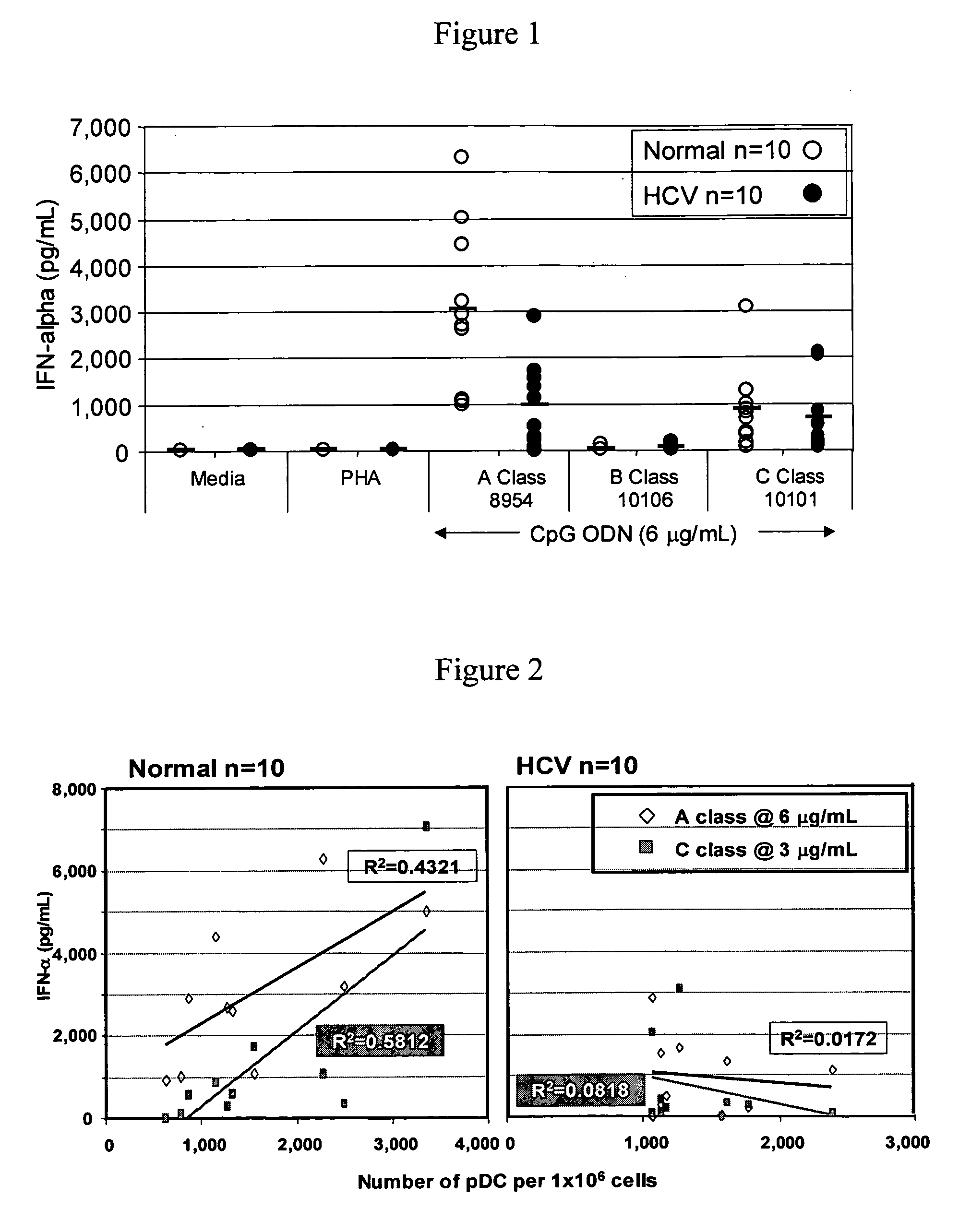
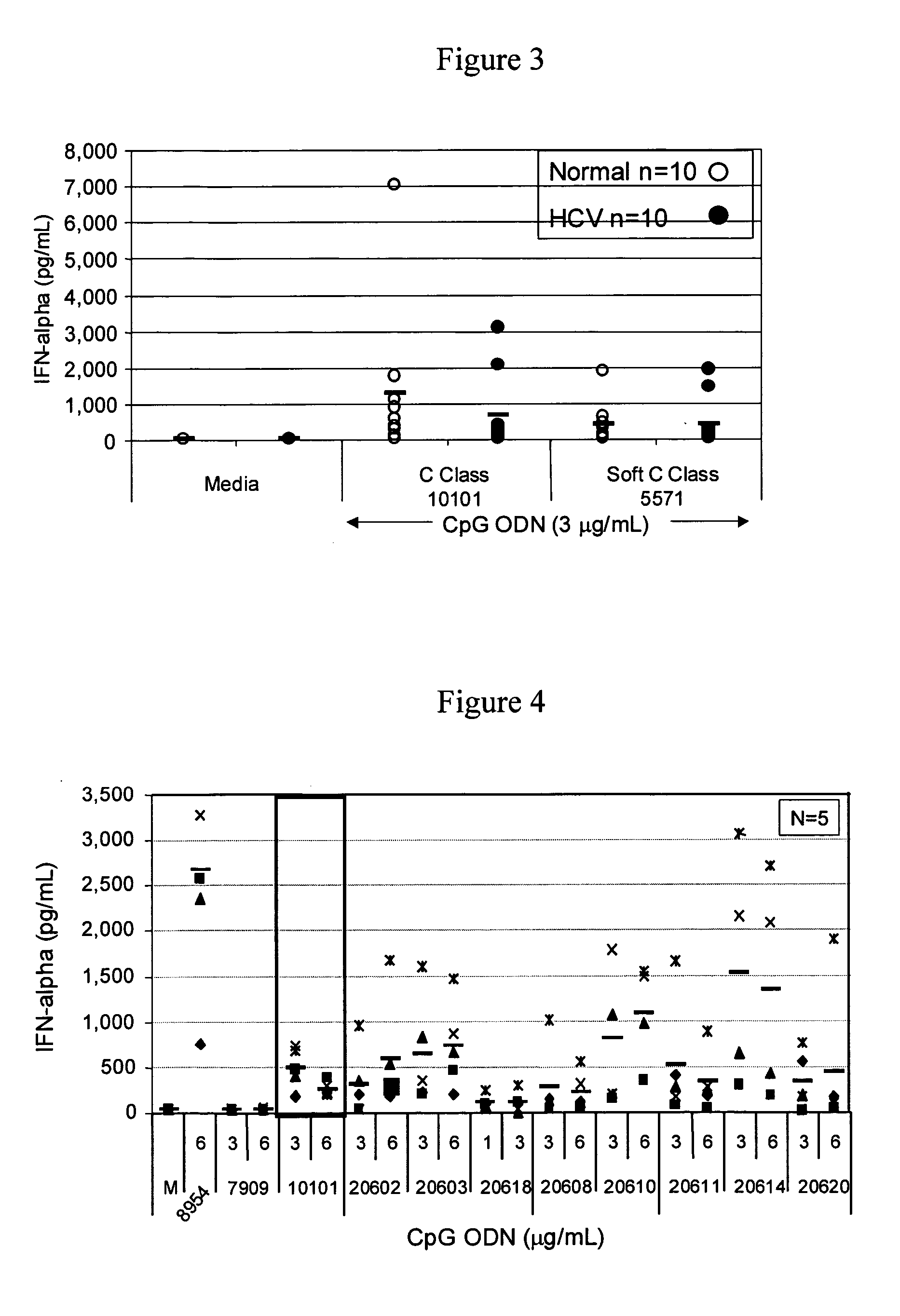
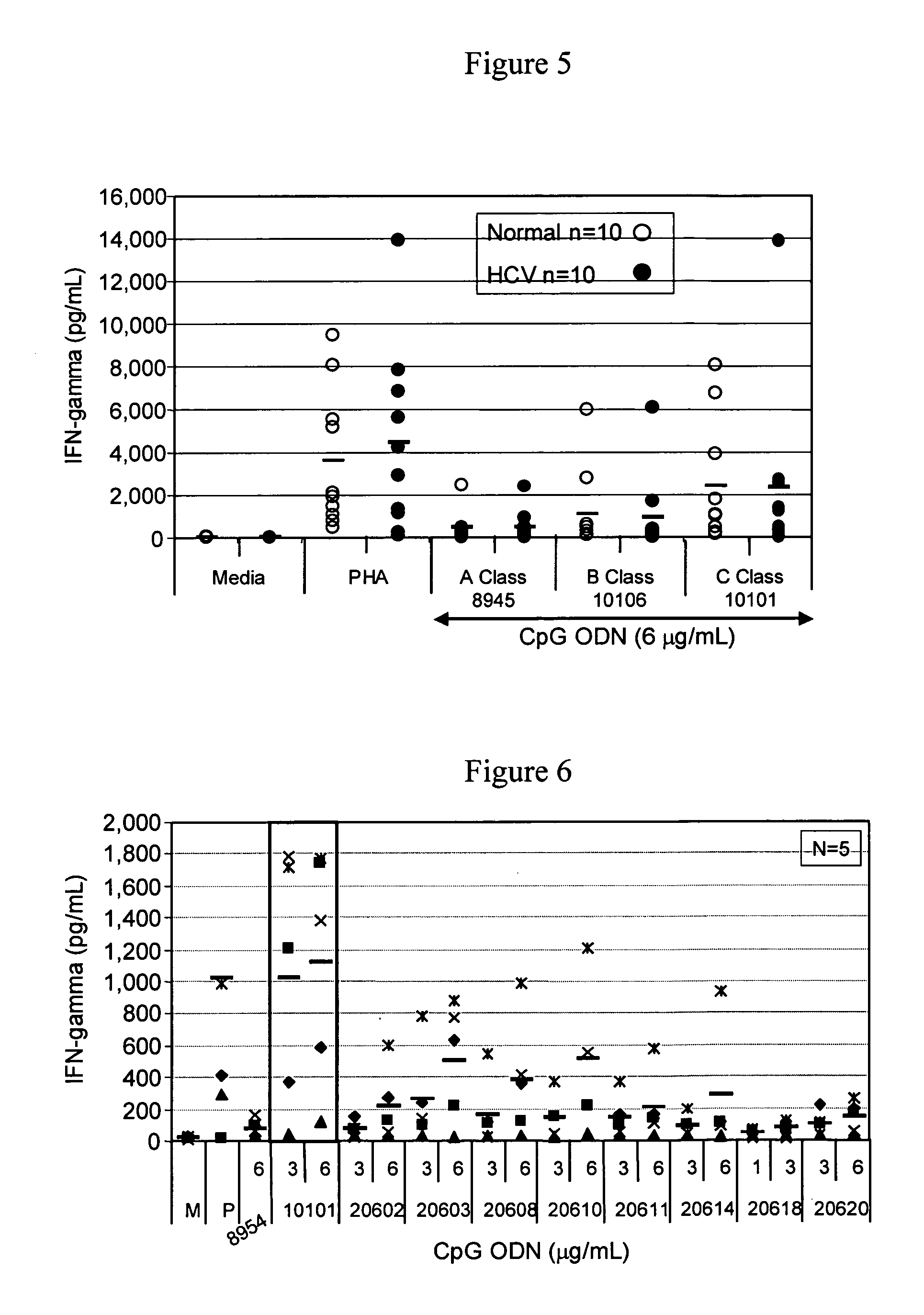
[0218] The purpose of this study was to evaluate the ability of different classes of CpG ODN to stimulate PBMC from HCV chronic carriers. PBMC were isolated from whole blood collected from normal, healthy volunteers and chronic carriers of HCV and the ability of the different classes CpG ODNs as well as soft and semi-soft molecules to stimulate B cell proliferation, cytokine secretion (IFN-g, TNF-α, IL-10 and IFN-α) and chemokine secretion (IP-10) in vitro was evaluated.
[0219] Also evaluated were the immune stimulatory effects of exogenous IFN-α-2b (Intron A) and Ribavirin, either alone, in combination with each other, and in combination with CpG ODN (B and C classes).
[0220] Materials and Methods
Oligonucleotides
[0221] All oligonucleotide stocks were resuspended in TE buffer at pH 8.0 (OmniPer®; EM Science, Gibbstown, N.J.). Dilutions of various ODNs were made in RPMI 1640 complete media (Gibco BRL, Grand Island, N.Y.) containing 10% heat inactivated, normal human AB serum (Wise...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com