Tetracyclic fused heterocyclic compound and use thereof as HCV polymerase inhibitor
a heterocyclic compound and tetracyclic technology, applied in heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of ineffective pharmaceutical agents, inability to eradicate the virus, and inability to effectively target the tumor by operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0843]
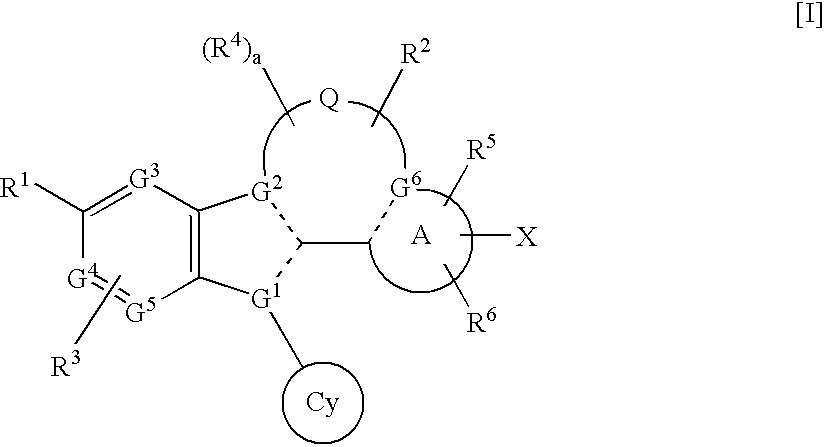
wherein Q10 is, for example, O or NH, Rc1 is a leaving group such as bromine atom, iodine atom, —OTf (trifluoromethylsulfonyloxy group) and the like, —B(ORc2)(ORc3) is —B(OH)2, 4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl group, ring A′ is ring A wherein G6 is carbon atom, and other symbols are as defined above.
[0844] Compound [2] can be obtained from commercially available compound [1] or compound [1] obtained by a conventional method and a boric acid ester.
[0845] As the boric acid ester, pinacolborane, bis(pinacolato)diboron and the like can be mentioned.
[0846] As a catalyst, palladium catalysts such as Pd(PPh3)4, Pd(dppb)Cl2, PdCl2(dppf)CH2Cl2, PdCl2(PPh3)2, Pd(OAc)2, PdCl2, palladium black, palladium carbon and the like can be mentioned.
[0847] As a base, strong bases such as ethylenediamine, sodium carbonate, barium hydroxide, potassium phosphate, cesium carbonate, sodium hydrogen carbonate, sodium tert-butoxide, potassium tert-butoxide, triethylamine, potassium acetat...
reference example 2
[0850]
wherein compound [4] is, for example, a compound wherein cycloalkyl group having 3 to 10 carbon atoms is substituted by oxo group, such as cyclopentanone, cyclohexanone and the like.
Step 1
[0851] Compound [5] can be obtained by reacting commercially available compound [3] or compound [3] obtained by a conventional method with compound [4] in the presence of a base, or under aldol reaction conditions.
[0852] As a base, preferably, sodium methoxide, sodium ethoxide, lithium diisopropylamide, sodium hydroxide, potassium hydroxide, sodium hydride and the like can be mentioned.
[0853] As a solvent, alcohol solvent such as methanol, ethanol and the like, THF, 1,4-dioxane, DMF (dimethylformamide), DMSO (dimethyl sulfoxide), DMA (dimethylacetamide), water and a mixed solvent thereof and the like can be mentioned.
[0854] As the reaction temperature, −20° C. to 120° C. is preferable.
[0855] In addition, for a reaction under acidic conditions, in a mixed solvent of acetic acid and pho...
reference example 3
[0857]
wherein Rc4 is carboxyl-protecting group such as methyl group, ethyl group, tert-butyl group, benzyl group and the like, Hal1 is halogen atom such as bromine atom, iodine atom and the like, and other symbols are as defined above.
Step 1
[0858] Compound [8] can be obtained by introducing a protecting group into a carboxyl group of compound [7] obtained by a conventional method or in the same manner as in Reference Example 2.
[0859] Where necessary, a protecting group may be introduced into a nitrogen atom of indole.
Step 2
[0860] Compound [9] can be obtained by halogenating compound [8] with a halogenating agent.
[0861] As the halogenating agent, bromine, N-bromosuccinimide, pyridine tribromide, dibromohydantoin, pyridinium hydrobromide perbromide, an iodide thereof and the like can be mentioned.
[0862] As a solvent, halogen solvents (dichloromethane, chloroform, carbon tetrachloride etc.), hydrocarbon solvents (toluene etc.), ether solvents (1,4-dioxane, DME (1,2-dimethoxye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com