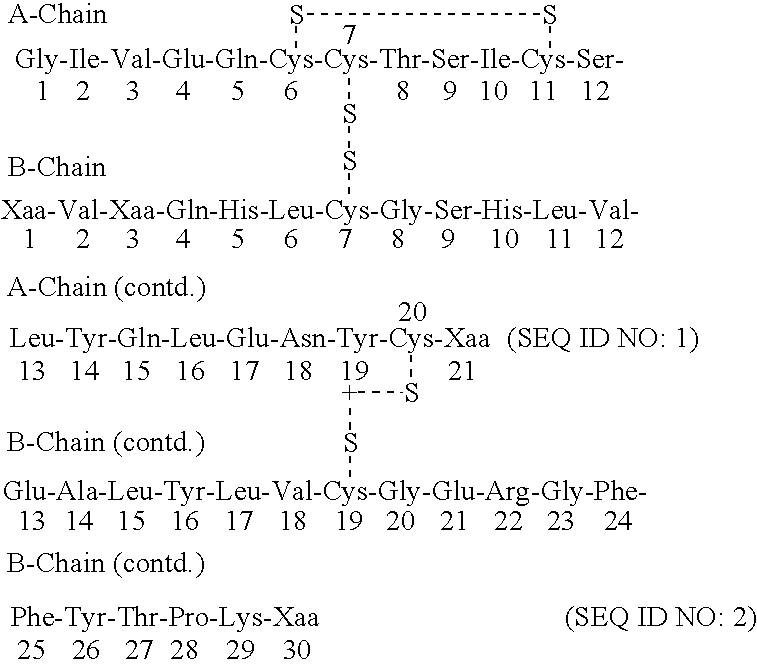
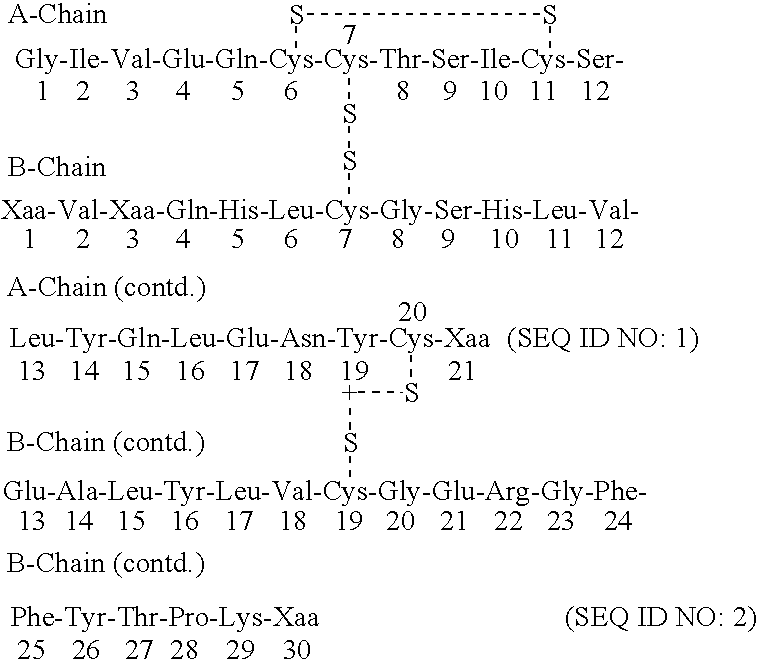
Acylated insulin
a technology of acylated insulin and insulin, which is applied in the field of human insulin derivatives, can solve problems such as tissue inflammation at the injection site, and achieve the effect of rapid onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of AlaA21 AspB3 Human Insulin Precursor from Yeast Strain yEA002 using the LaC212spx3 Signal / Leader.
[0397] The following oligonucleotides were synthesized:
#98(SEQ ID NO:3)5′-TGGCTAAGAGATTCGTTGACCAACACTTGTGCGGTTCTCACTTGGTTGAAGCTTTGTACTTGGTTTGTGGTGAAAGAGGTTTCTTCTACACTCCAAAGTCTGACGACGCT-3′ (AspB3)#128(SEQ ID NO:4)5′-CTGCGGGCTGCGTCTAAGCACAGTAGTTTTCCAATTGGTACAAAGAACAGATAGAAGTACAACATTGTTCAACGATACCCTTAGCGTCGTCAGACTTTGG-3′ (AlaA21)#126(SEQ ID NO:5)5′-GTCGCCATGGCTAAGAGATTCGTTG-3′ (AspB3)#16(SEQ ID NO:6)5′-CTGCTCTAGAGCCTGCGGGCTGCGTCT-3′
[0398] The following Polymerase Chain Reaction (PCR) was performed using the Gene Amp PCR reagent kit (Perkin Elmer, 761 Main Avewalk, Conn. 06859, USA) according to the manufacturer's instructions. In all cases, the PCR mixture was overlayed with 100 μl of mineral oil (Sigma Chemical Co., St. Louis, Mo., USA). [0399] 2.5 μl of oligonucleotide #98 (2.5 pmol) [0400] 2.5 It of oligonucleotide #128 (2.5 pmol) [0401] 10 μl of 10×PCR buffer [0402] 16 μ...
example 2
Synthesis of AlaA21 ThrB3 Human Insulin Precursor from Yeast Strain yEA005 using the LaC212spx3 Signal / Leader.
[0412] The following oligonucleotides were synthesized:
#101(SEQ ID NO:7)5′-TGGCTAAGAGATTCGTTACTCAACACTTGTGCGGTTCTCACTTGGTTGAAGCTTTGTACTTGGTTTGTGGTGAAAGAGGTTTCTTCTACACTCCAAAGTCTGACGACGCT-3′ (ThrB3)#128(SEQ ID NO:4)5′-CTGCGGGCTGCGTCTAAGCACAGTAGTTTTCCAATTGGTACAAAGAACAGATAGAAGTACAACATTGTTCAACGATACCCTTAGCGTCGTCAGACTTTGG-3′ (AlaA21)#15(SEQ ID NO:8)5′-GTCGCCATGGCTAAGAGATTCGTTA-3′ (ThrB3)#16(SEQ ID NO:6)5′-CTGCTCTAGAGCCTGCGGGCTGCGTCT-3′
[0413] The DNA encoding AlaA21 ThrB3 human insulin precursor was constructed in the same manner as described for the DNA encoding AlaA21 AspB3 human insulin precursor in Example 1. The DNA sequence encoding the LaC212spx3 signal / leader / AlaA21 ThrB3 human insulin precursor complex and the amino acid sequence thereof are SEQ ID NOS. 23, 24 and 25. The plasmid pEA8.1.1 was shown to contain the desired sequence, transformed into S. cerevisiae strain M...
example 3
Synthesis of GlyA21 AspB3 Human Insulin Precursor from Yeast Strain yEA007 using the LaC212spx3 Signal / Leader.
[0414] The following oligonucleotides were synthesized:
#98(SEQ ID NO:3)5′-TGGCTAAGAGATTCGTTGACCAACACTTGTGCGGTTCTCACTTGGTTGAAGCTTTGTACTTGGTTTGTGGTGAAAGAGGTTTCTTCTACACTCCAAAGTCTGACGACGCT-3′ (AspB3)#127(SEQ ID NO:9)5′-CTGCGGGCTGCGTCTAACCACAGTAGTTTTCCAATTGGTACAAAGAACAGATAGAAGTACAACATTGTTCAACGATACCCTTAGCGTCGTCAGACTTTGG-3′ (GlyA21)#126(SEQ ID NO:5)5′-GTCGCCATGGCTAAGAGATTCGTTG-3′ (AspB3)#16(SEQ ID NO:6)5′-CTGCTCTAGAGCCTGCGGGCTGCGTCT-3′
[0415] The DNA encoding GlyA21 AspB3 human insulin precursor was constructed in the same manner as described for the DNA encoding AlaA21 AspB3 human insulin precursor in Example 1. The DNA sequence encoding the LaC212spx3 signal / leader / GlyA21 AspB3 human insulin precursor complex and the amino acid sequence thereof are SEQ ID NOS. 26, 27 and 28. The plasmid pEA1.5.6 was shown to contain the desired sequence, transformed into S. cerevisiae strain MT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| mean particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap