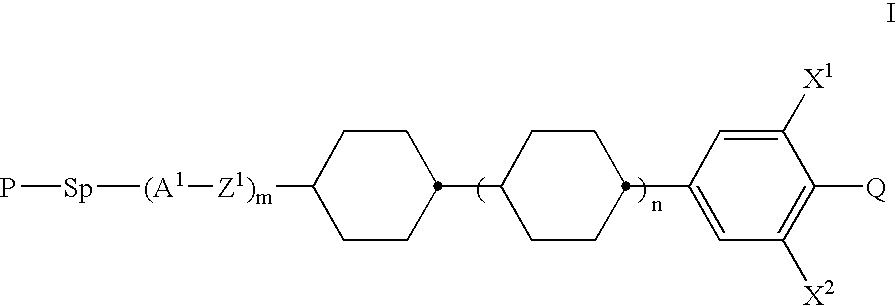
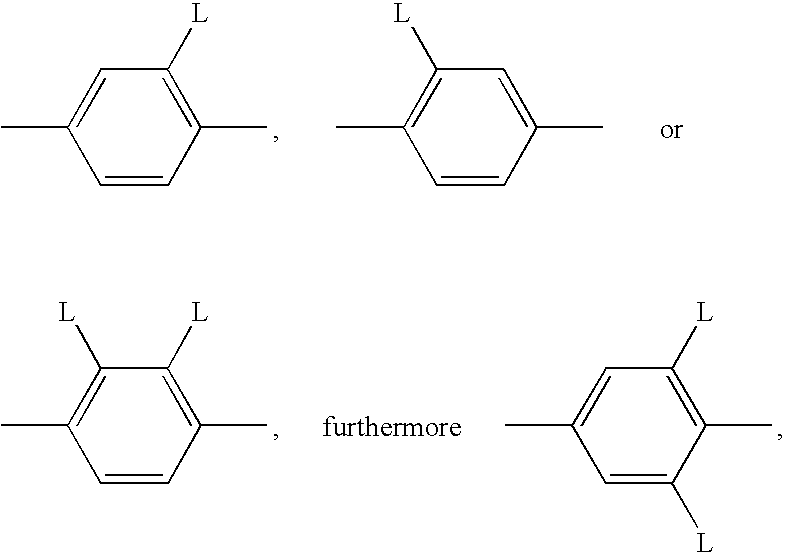
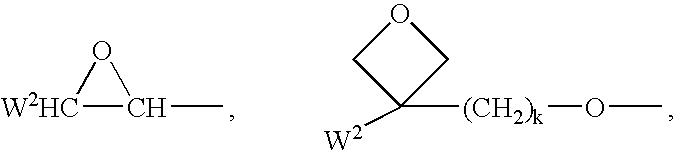Polymerizable mesogenic cyclohexyl derivatives
a technology of cyclohexyl derivatives and mesogenic cyclohexyls, which is applied in the field of new polymerizable mesogenic or liquid crystalline compounds, can solve the problems of poor solubility, high melting point, and high value of prior art compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0127]
is prepared as follows:
[0128] Step 1:
[0129] Step 2:
[0130] Step 3:
[0131] Step 4:
Steps 1-4 have been described in the literature.
[0132] Step 5:
[0133] 36.6 g CCU-on-F (98% trans), 44.1 g cer(lIl)chlorid heptahydrate (99.9%, Aldrich) are dissolved in 300 ml methanol and 150 ml THF. At 0-10° C. 2.081 g solid NaBH4 (96%, Merck) is added portionwise. After the reaction is finished (5-20 min, DC-control) the mixture is quenched with 70 ml aq. ammonium chloride solution and 300 ml toluene are added. The mixture is filtered, the organic phase separated and the aqueous phase extracted with 100 ml toluene 3 times. The org. phases are dried, the solvent is evaporated and the residue recrystallized from heptane / toluene to give 32.6 g of a white solid (98.1% all-trans).
[0134] Step 6:
[0135] In a 500 ml four-neck flask 7.48 g DCC in 50 ml dichloromethane are added under a nitrogen atmosphere to a solution of 10.0 g CCU-ol-F, 117 mg DMAP and 7.36 g succinic acid mono-[2-(2-meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| anisotropic | aaaaa | aaaaa |
| mechanical properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com