Orbital atherectomy device guide wire design
a technology for orbital atherectomy and guide wires, which is applied in the field of medical devices, can solve the problems of bending failure of ptfe tubing, premature failure of oad and/or guide wires, and segment difficulty in extraction, so as to reduce the trauma to the vessels, reduce trauma, and reduce the effect of trauma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
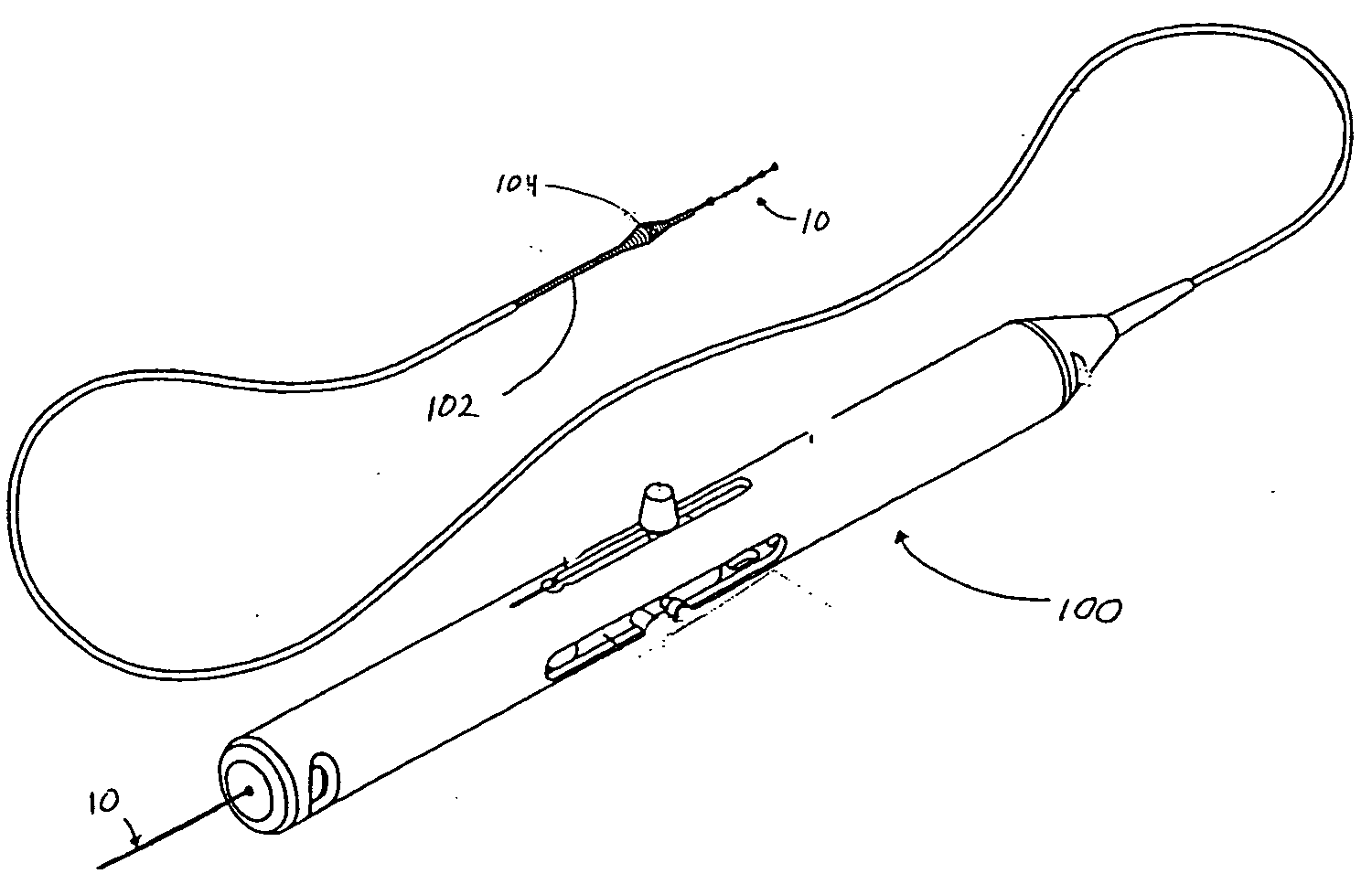
[0018] The present invention relates to a novel design for a guide wire that is generally illustrated throughout the figures as guide wire 10. The figures illustrate the guide wire 10 in particular embodiments and, in some figures, in conjunction with particular medical devices for ease of description and understanding. The figures are not intended to limit the possible applications of the present invention. The scope of the invention will be defined by the claims and will be understood by those skilled in the art upon review of the specification and figures.
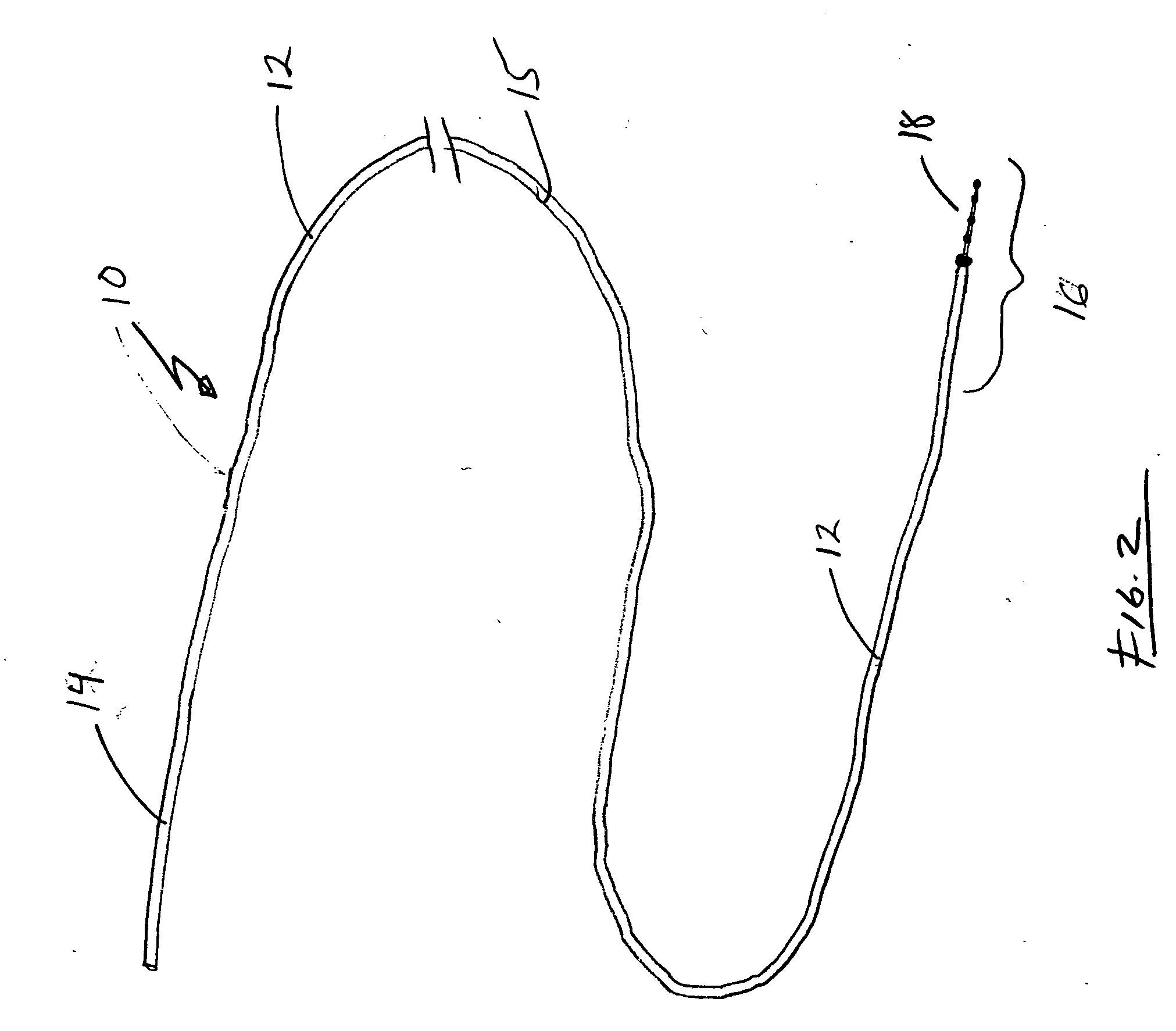
[0019] Turning to FIG. 2, guide wire 10 is generally configured to permit distal region 16 of guide wire 10 to be positioned, relatively atraumatically, at a desired location within a patient. Accordingly, guide wire 10 is flexible and includes an atraumatic tip 18 to prevent damage to tissues as the guide wire is advanced.
[0020] Guide wire 10 generally includes an elongated shaft 12 having a proximal region 14, a central regi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com