Methylphenidate derivatives and uses of them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0105] Whole blood was drawn from GR283, a human volunteer with known allergies, into a glass vacutainer tube containing no anticoagulant. This blood was allowed to clot, and the serum was removed by centrifugation and then heat inactivated by placing it in a water bath at 56° C. for 30 minutes. Whole blood from GR283 was also drawn into a glass vacutainer tube containing heparin and used for peripheral blood lymphocytes (PBL) isolation as follows. Whole blood was layered over room temperature Histopaque 1077 solution and centrifuged at 2000 rpm for 15 minutes at room temperature. Cells at the plasma-Histopaque interface were then removed and washed with culture medium (IMDM medium with 10% heat-inactivated GR283 serum plus 1% penicillin / streptomycin) at 37° C.
[0106] The compound of formula II (see above) and methylphenidate (both obtained from Dr. Jeffrey D. Winkler, University of Pennsylvania, Philadelphia, Pa.) in culture medium were added to wells of a 96-well plate to give fin...
example 2
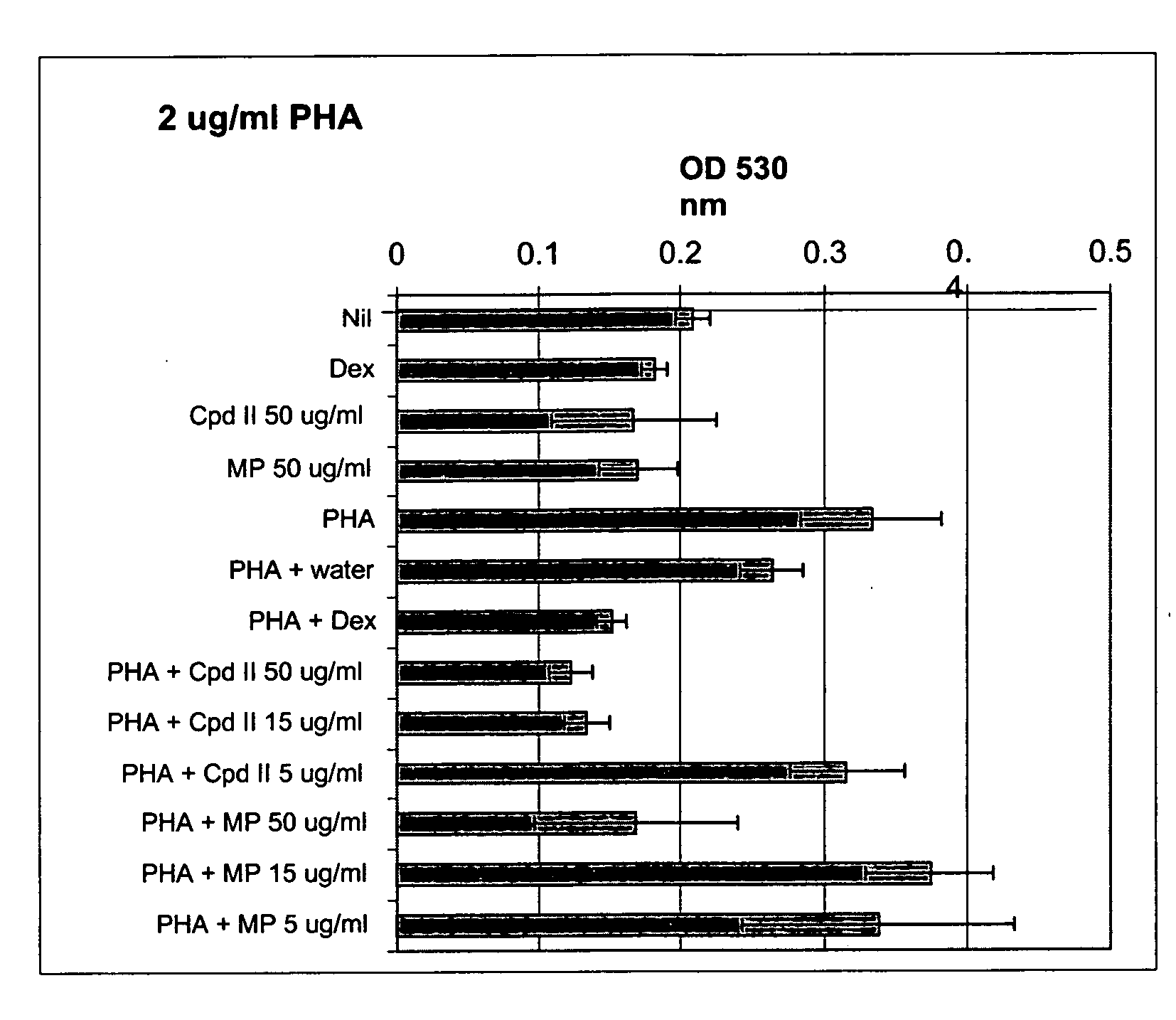
[0109] Whole blood was drawn from GR467, a human volunteer with known allergies, and processed as described in Example 1 to give heat-inactivated serum and PBL. The compound of formula II and methylphenidate in culture medium (made using heat-inactivated GR467 serum) were added to wells of a 96-well plate to give final concentrations of 5 μg / ml, 15 μg / ml and 25 μg / ml of the compound of formula II and 15 μg / ml methylphendiate. Water and dexamethasone (final concentration of 10 μM) were used as controls. Then, GR467's PBL in culture medium were added to the wells to give a final concentration of 150,000 cells per well, and the plates were incubated at 37° C., 5% CO2 for 24 hours. After this incubation, PHA was added to give a final concentration of 2 μg / ml, final total volume of 200 μl / well, and the cells were incubated for an additional 72 hours at 37° C., 5% CO2. All cultures were performed in triplicate.
[0110] At the end of this incubation, cell proliferation was determined as des...
example 3
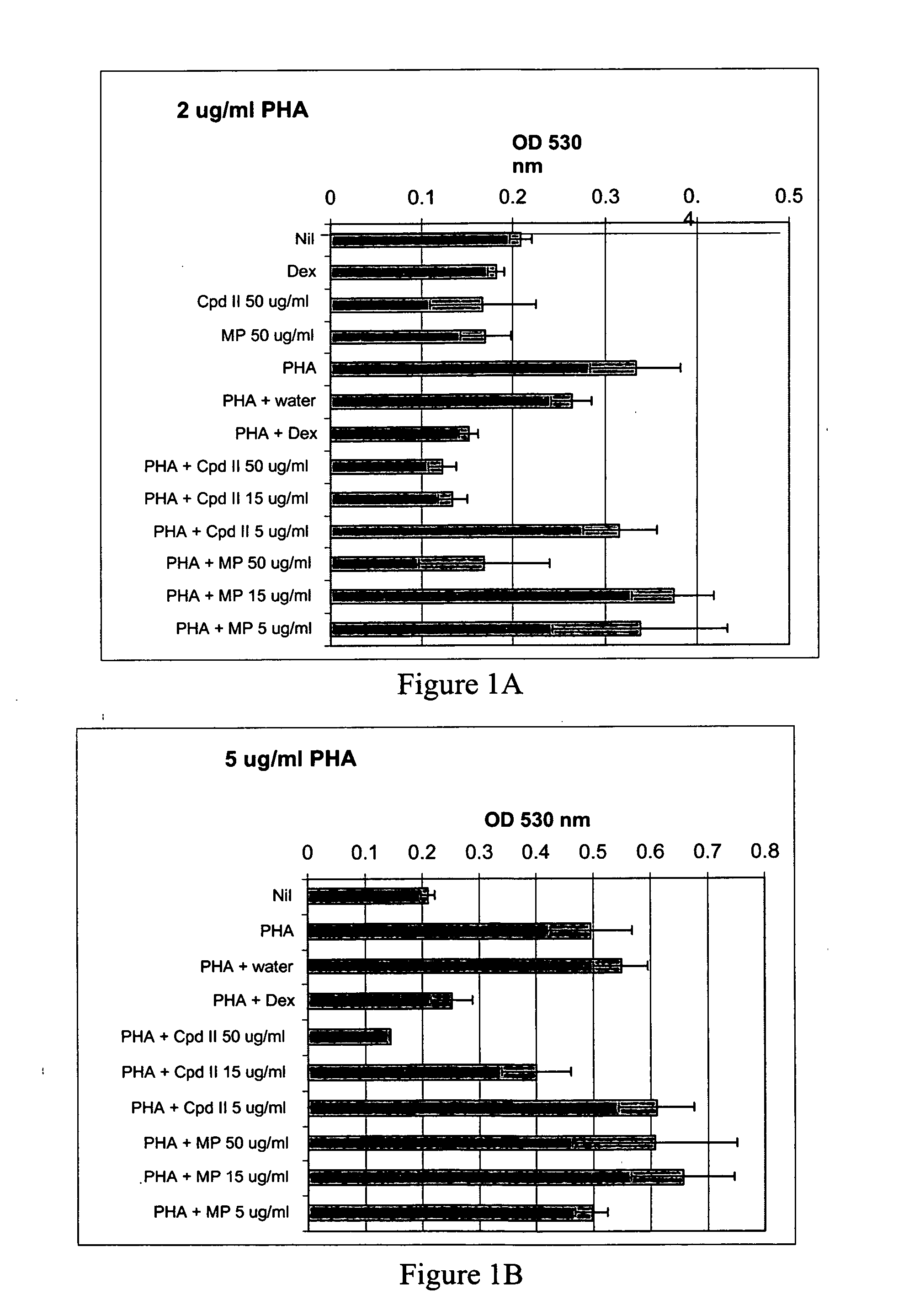
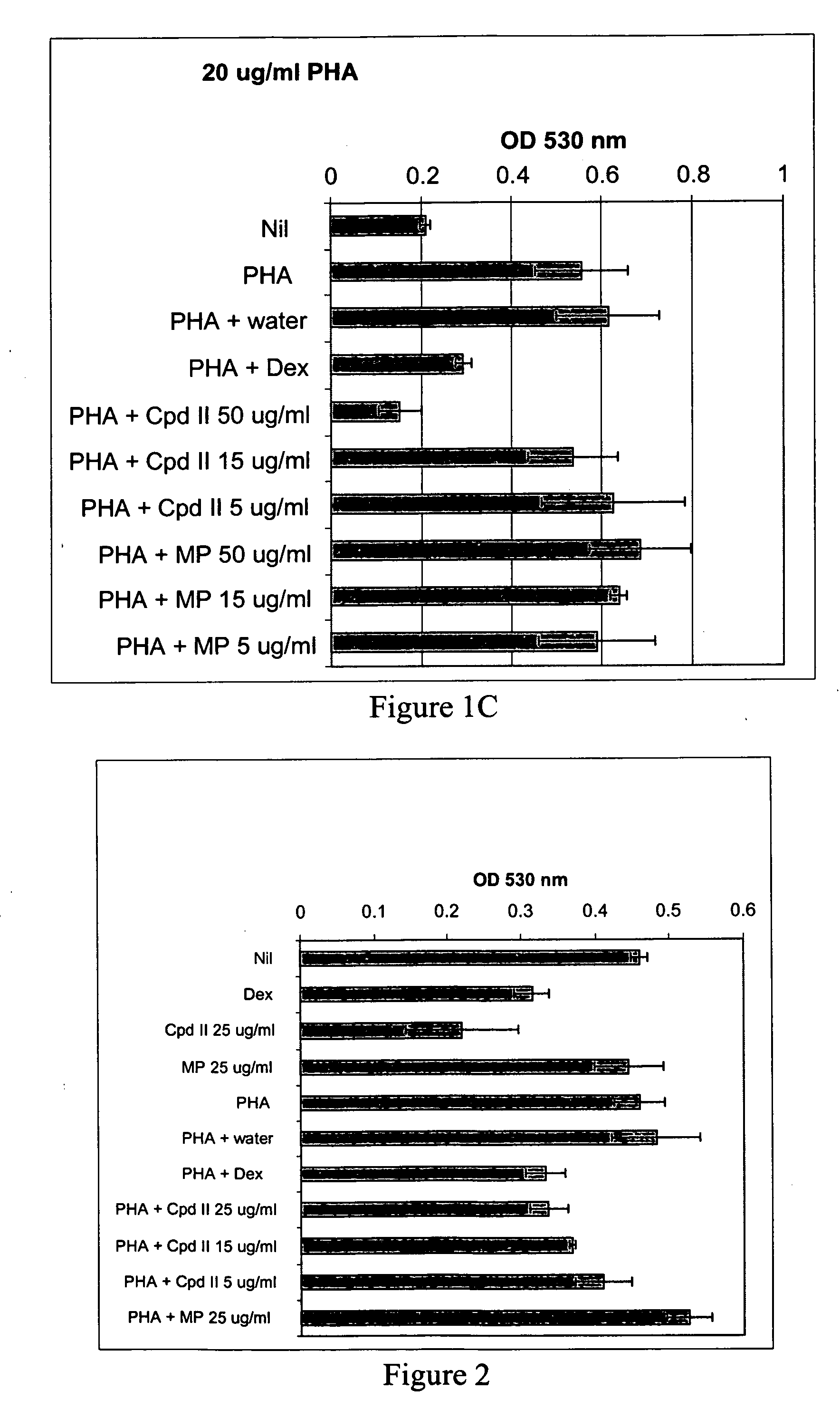
[0117] Whole blood was drawn from GR191, a normal human volunteer, and processed as described in Example 1 to give heat-inactivated serum and PBL. The compound of formula II and methylphenidate in culture medium (made using heat-inactivated GR191 serum) were added to wells of a 96-well plate to give final concentrations of 5 μg / ml, 15 μg / ml, 25 μg / ml and 50 μg / ml of the compound of formula II and 50 μg / ml methylphendiate. Water, mouse nerve growth factor (Upstate Biotechnology, Inc) (NGF) (final concentration of 250 ng / ml) and dexamethasone (final concentration of 10 μM) were used as controls. Then, GR191's PBL in culture medium were added to the wells to give a final concentration of 150,000 cells per well, and the plates were incubated at 37° C., 5% CO2 for 24 hours. After this incubation, PHA was added to give final concentrations of 2 μg / ml and 5 μg / ml, final total volume of 200 μl / well, and the cells were incubated for an additional 72 hours at 37° C., 5% CO2. All cultures were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com