Sustained release ophthalmological device and method of making and using the same
a technology of ophthalmological devices and suture release, which is applied in the field of ophthalmologic implants, can solve problems such as bulky intravitreal implants, and achieve the effect of reducing post-operative prescription drug costs and associated health care needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
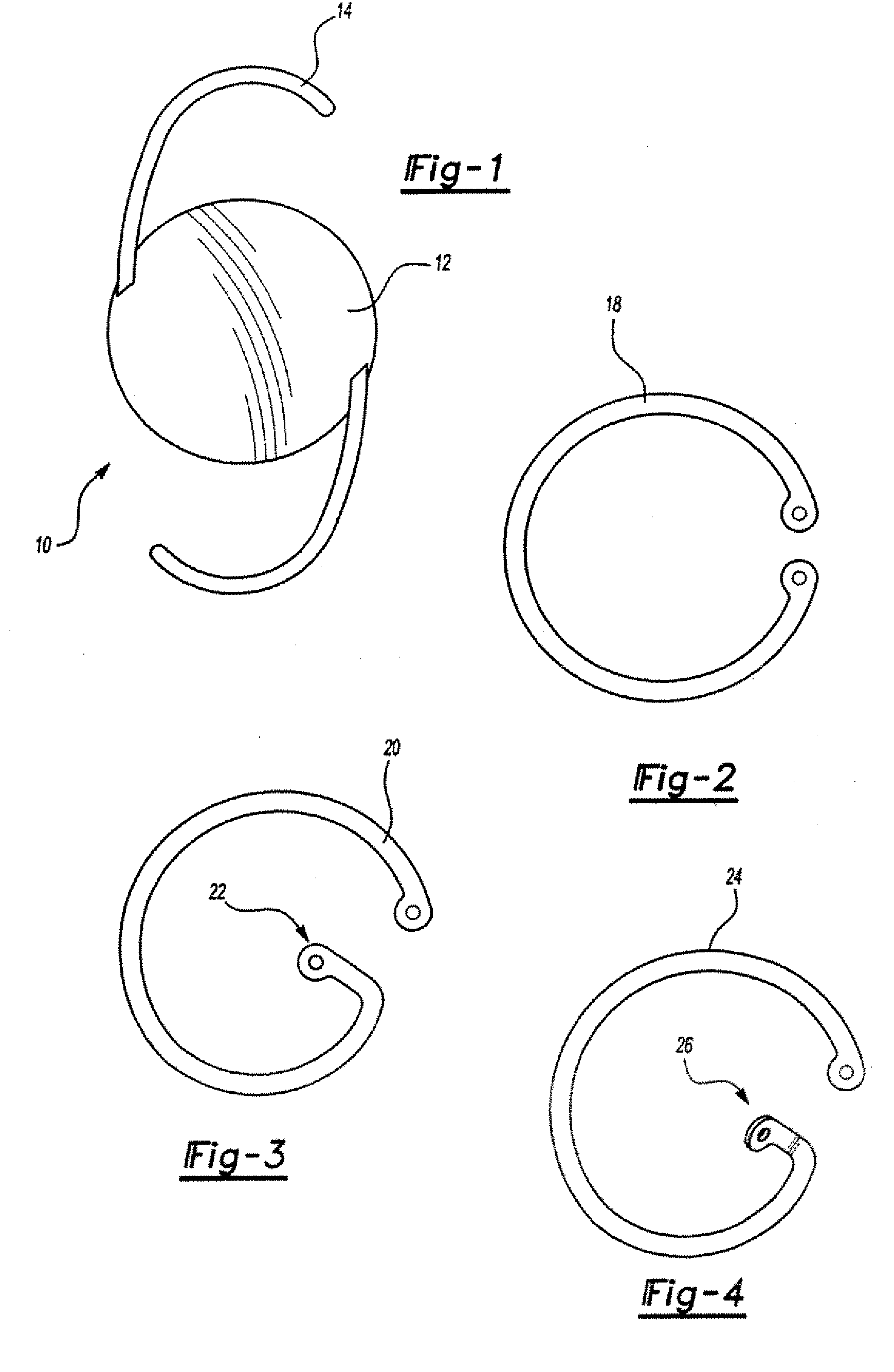
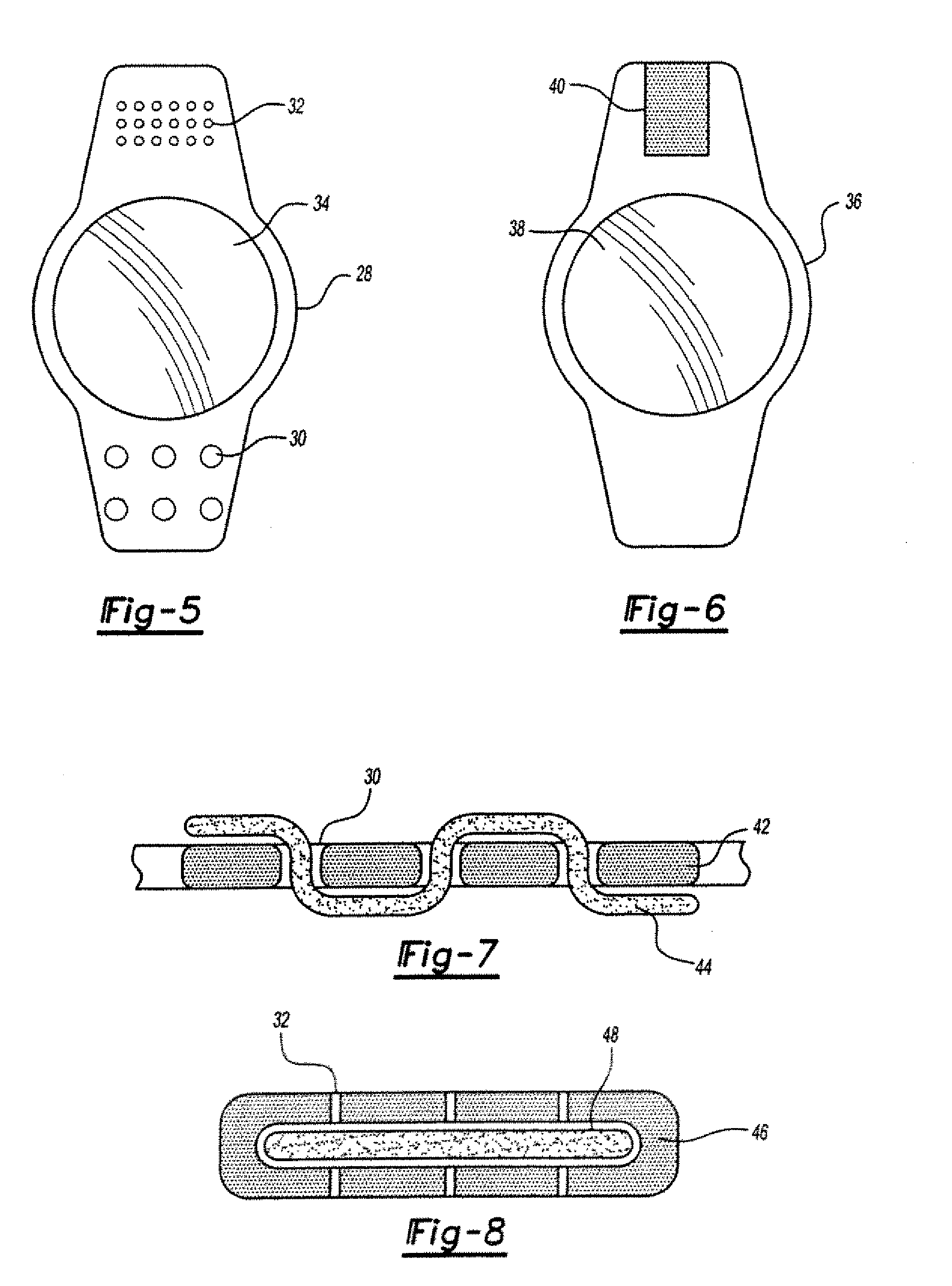
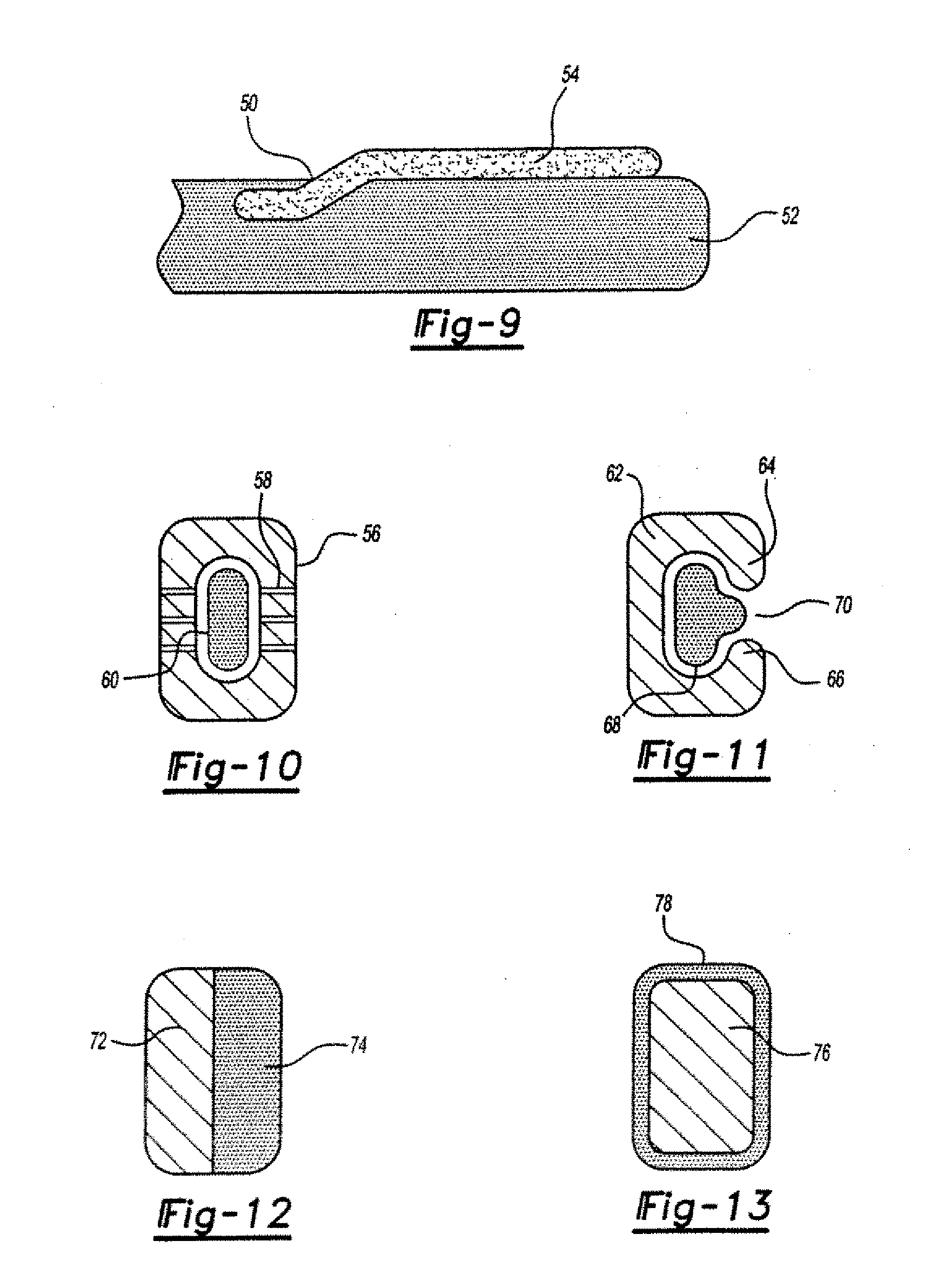
[0026] In general, the ophthalmologic implants of the present invention will include at least one member, such as a filament, particularly one that is made of a biocompatible material that supports a pharmaceutical agent, and more preferably a pharmaceutical agent that is carried by a sustained release medium material, such as one selected from a bioerodible material, a biodegradable material, a bioavailable material or a mixture thereof.
[0027] The pharmaceutical agent herein may be a single agent, an admixture of agents, or multiple agents applied in simultaneous or serial coatings or layers. The pharmaceutical agent preferably is selected from an antibiotic, an anti-inflammatory, an antiglaucomatous, a steroid, or a combination thereof. By way of illustration (but without limitation to the employment of other unlisted agents), specific examples of pharmaceutical agents include 5-fluorouracil (5-FU), cyclosporine A (CsA), vancomycin, ganciclovir, fluocinolone acetonide, dexamethas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com